Case 12
A 73-year-old woman, s/p ICD implanted for recurrent ventricular tachycardia (VT) associated with dilated cardiomyopathy (DCM) 9 months ago, was admitted after receiving several electrical
A 41-year-old man with Limb-Girdle muscular dystrophy (LGMD) was brought to the Emergency Department complaining of having intermittent chest tightness, cold sweating, and shortness of breath (SOB) for one day and a near syncope episode two hours prior. Past medical history is significant for hypertension and chronic hepatitis B for a few years and an uneventful PTCA with stenting for left anterior descending artery (LAD) stenosis (single vessel coronary heart disease) presenting as non-ST elevation myocardial infarction (NSTEMI) two months before. He was otherwise in no acute distress. BT/PR/RR measured 35.6 ºC/59 ppm/16 cpm. BP was 111/66 mmHg. He walked in a waddling gait.* There was no JVD, but there were RV and LV lifts with a Gr 2/6 systolic murmur at the apical area (displaced PMI one cm left to the left midclavicular line). The lungs were clear. The liver was two finger-breath palpable below the right costal margin. Notably, pelvic, shoulder, and lower limb muscle groups were relatively atrophic. ECG showed sinus bradycardia at 54/min, with findings suggesting biatrial and biventricular enlargement, intraventricular conduction defect (IVCD), old high lateral wall MI, and anterior and inferolateral wall subendocardial myocardial ischemia or infarction. Chest X-ray revealed cardiomegaly (LVH), RA and LA enlargement, pulmonary hypertension, and mild pulmonary congestion (heart failure [HF]). Pertinent laboratory data included Hb 16.1 g/dL, Hct 48%, Plt 198 K/ul, WBC 176300 /ul, Troponin-I 0.06 (N <0.04) ng/ml, BUN 17 mg/dL, Cr 0.51 mg/dL, SGPT 51 (N <40) U/l, Na 134 mM, K 4.4 mM, CPK 1,388 (N 10-120) U/L, CPK-MB 41 (N 5 to 25) U/L. Under the impression of acute coronary syndrome presenting with another episode of NSTEMI, the care team performed an emergent coronary arterial angiography, which revealed all three vessels, including the stented LAD, were patent. Echocardiography confirmed four-chamber dilatation, normal wall thickness, and global hypokinesis (impaired LV contractility) with moderate MR and TR. A 24-hour Holter monitoring captured no significant arrhythmias, particularly no long pause, A-V block, or tachyarrhythmias. Subsequent persantin-thallium scan exhibited severe ischemia or infarction in the apex, apical, anterior wall, apical inferior wall, and basal inferolateral wall and mild to moderate ischemia in the lateral wall. As such, NSTEMI likely was related to LGMD, albeit speculative, a process called “spontaneous myocarditis.” To search for a non-cardiac cause accounting for the highly elevated CPK level, the care team performed an incisional muscle biopsy of the left biceps, which revealed a severe grouping of atrophic myofibers with some regenerative myofibers, indicating muscular dystrophy with neurogenic changes. A neurological study showed bilateral femoral cutaneous neuropathies and bilateral lumbosacral radiculopathies. Therefore, the patient seemed also to have a flare-up of spontaneous myositis with neuropathy. He received aspirin, clopidogrel, enoxaparin carvedilol, and valsartan throughout the course. At last, the care team referred the patient to cardiology and neurology services for further assessment (e.g., genetic study) and follow-up care.
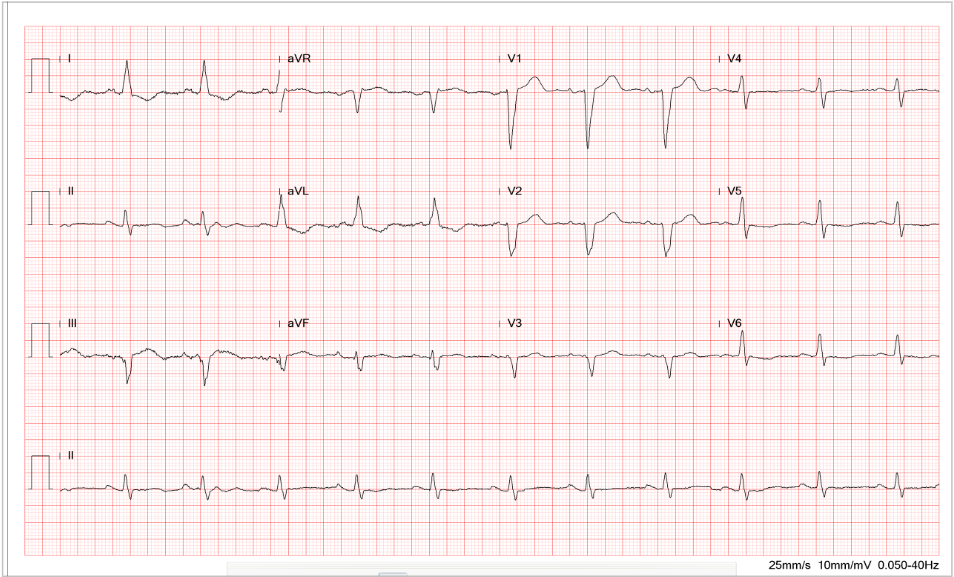
Sinus bradycardia at 54/min
Tall P in leads II (>2.5 mV), III, and aVF, and P having a prominent negative deflection in V1
and V2, suggestive of RA and LA enlargement, respectively.
Fragmented QRS with an initial slurring and intraventricular conduction delay (IVCD) (QRSd
120 msec)
Q wave in leads I and aVL representative of old high lateral wall MI
Tall R wave in V1 and V2 with initial slurring suggesting possible true posterior wall MI, r/o
RVH
Equalization of R and S in V3-V5 alongside tall R in V1 and V2, suggestive of biventricular enlargement.
T wave inversion in leads V1-V4 and ST-segment depression in leads II, III, aVF, and V4-V6
suggestive of anterior and inferolateral wall myocardial ischemia.

Emphysematous lungs (COPD)
Cardiomegaly with LA enlargement
Aortic knob calcification
Prominent PA indicative of PH
Pulmonary congestion suggesting HF
Pulmonary infiltrates likely due to pneumonia
Mitral ring calcification.
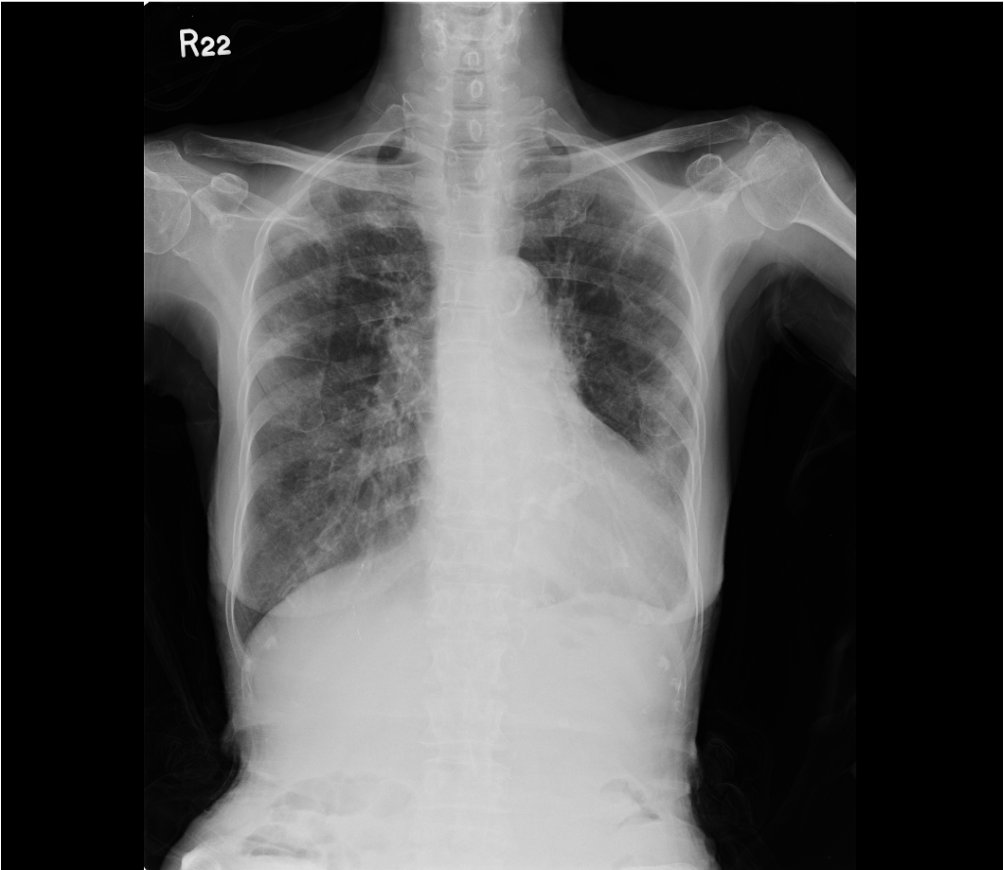
Cardiomegaly suggestive of LVH
RA enlargement (right-ward shift of the RA border)
LA enlargement (widened carina with double density and bulged left-sided
cardiac wall below PA)
Prominent PA suggestive of pulmonary hypertension
Slight increase in pulmonary vascularity (mild HF cannot be excluded
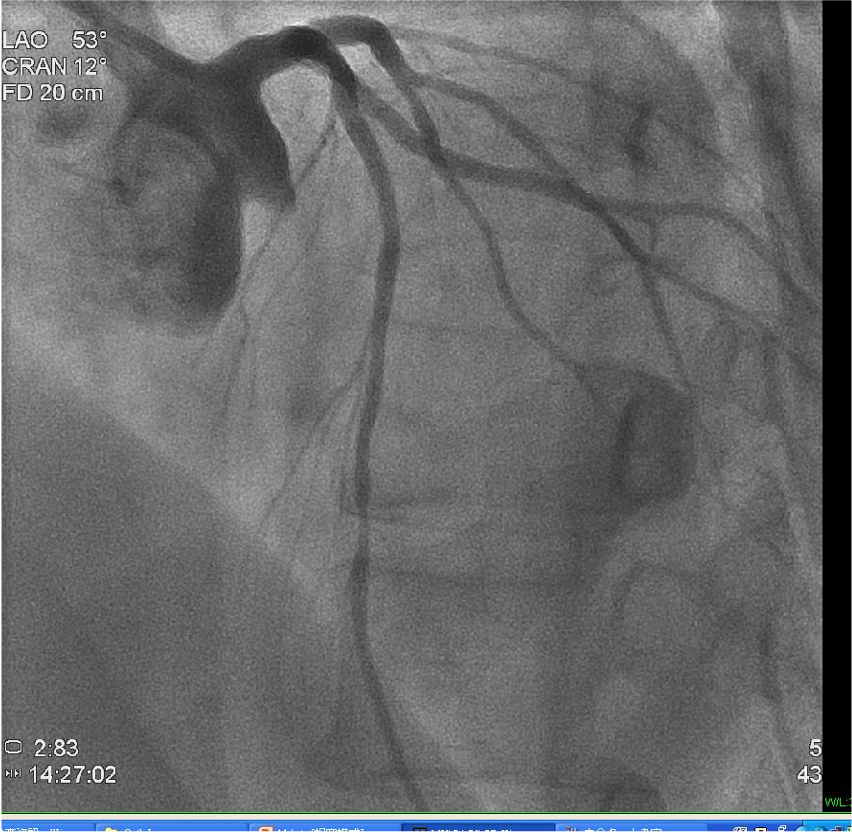
LAD mid-portion stenosis 50%. Other vessels are free of significant lesions.
RCA not shown.

Four-chamber dilatation (LA 52.20 mm in diameter, LA/Ao ratio 1.96)
Normal wall thickness with global hypokinesis (impaired LV contractility)
Moderate MR and TR and mild PR
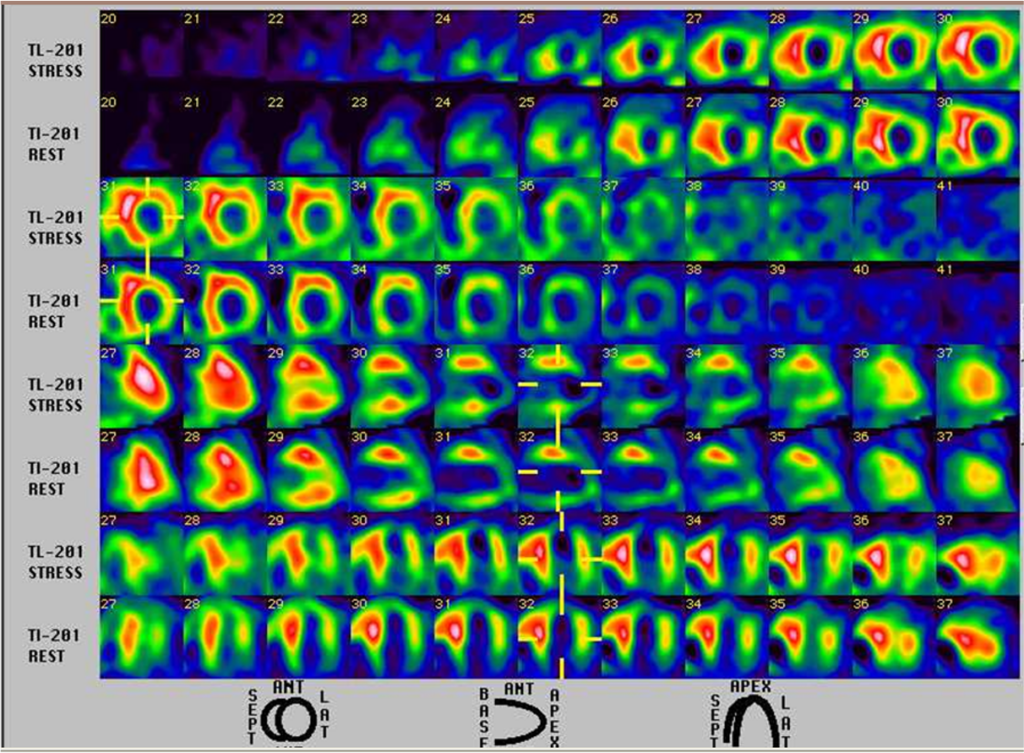
Severe ischemia or infarction in the apex, apical anterior wall, apical inferior wall, and basal inferolateral wall.
Mild to moderate ischemia in the lateral wall.
This case presents a complex ECG and intricate laboratory and imaging findings. On the part of ECG:
Supportive evidence of the above ECG narrative is as follows: Chest X-ray shows findings favoring RA, LA enlargement, and LVH with pulmonary hypertension. RVH is usually seen in the lateral view, which is unavailable in the present case. Echocardiography exhibited four-chamber dilatation with normal wall thickness and reduced LV systolic function, aligning with dilated cardiomyopathy associated with muscular dystrophy*** like LGMD. The persantin-thallium scan illustrates active myocardial ischemia in the apical anterior, apical inferior, and basal inferolateral wall.
LGMD refers to conditions primarily affecting the voluntary muscles around the hips and shoulders (limb-girdle muscles). However, the involvement of other organ systems, such as the heart, is not uncommon and adds to the complexity of this disorder. Many subtypes of LGMD, like LGMD R4 (beta-sarcoglycan-related) and LGMD R9 (FKRP-related), have associated cardiac involvement manifesting as dilated cardiomyopathy and cardiac conduction disturbances, as illustrated in the present case. Some patients with cardiac involvement don’t have overt symptoms. Dilated cardiomyopathy can lead to HF if untreated, and cardiac arrhythmias, particularly A-V block and ventricular tachyarrhythmias, can cause fainting spells, palpitations, and sudden death.
The markedly elevated CPK (1388 [N 10-120] U/L) relative to a slight increase in CPK-MB (41 [N 5 to 25] U/L) is intriguing. The CPK enzyme is found in the heart, skeletal muscle, and brain. It gets elevated in situations like MI, muscle trauma, vigorous exercise, or certain neuromuscular diseases like Duchenne and Becker muscular dystrophy and LGMD. CPK-MB, a subtype of CPK, can be found in the heart muscle. However, other muscles also contain CPK-MB, so tests for cardiac troponin (specific to the heart) are the tests of choice. In the present case, the troponin-I is slightly elevated (0.06 [N <0.04] ng/m), and the persantin-thallium scan has shown extensive myocardial ischemia beyond the LAD territory. Thus, in light of patent coronary arteries (including the stented LAD), spontaneous or idiopathic viral myocarditis and residual ischemia should be considered in this patient with LGMD.
Nevertheless, the disproportional increase in CPK (1,388 [N 10-120] U/L) relative to the CPK-MB (41 [N 5 to 2) U/L) level suggests that the CPK elevation source is primarily non-cardiac (e.g., muscle trauma, myositis, vigorous exercise, or rhabdomyolysis). Other medical conditions like hypothyroidism, hyperthyroidism, and hypokalemia can also cause elevation of CPK levels. In the present case with LGMD, spontaneous myositis (inflammation of muscles) cannot be excluded despite the lack of typical signs and symptoms, i.e., muscle pain, tenderness, and low-grade fever. The symptoms of chest tightness, SOB, and near syncopal episodes are more in line with cardiac origin. On the other hand, leukocytosis (WBC 176300/ul) is nonspecific, as it can occur in either myocarditis or myositis. Spontaneous myositis occurrences may lead to a more severe and rapid progression of the underlying muscular dystrophy. Monitoring for CPK and symptoms, if present, is crucial. Always consider these in the context of the patient’s overall clinical picture and seek appropriate consultation to confirm the diagnosis whenever myositis is suspected.
Interestingly, the patient is also noted to have a neurogenic abnormality, i.e., at least bilateral femoral cutaneous neuropathies and bilateral lumbosacral radiculopathies. CPK is a nonspecific marker of muscle damage, as it can be elevated in neuropathic and myopathic processes. Differentiation between the two can be challenging. However, myopathy is a condition that affects the muscles themselves. In myopathy, CPK levels are usually very high, and there is often proximal muscle weakness (weakness in the muscles closest to the body’s core). There may also be muscle tenderness and contractures (permanent shortening of muscles or joints). In contrast, neuropathy is a condition that affects the nerves that control the muscles. In neuropathy, CPK levels are usually normal or only slightly elevated. Distal muscle weakness (weakness in the muscles farthest from the body’s core) and sensory symptoms (such as tingling or numbness) are often present. As such, the increased CPK is likely caused much more by myositis than neuropathy in the present case.
Spontaneous myocarditis is an inflammation of the heart muscle without any known cause or trigger. It can indeed be observed in patients with muscular dystrophy***, primarily in Duchenne and Becker muscular dystrophy (inherited X-linked, recessive disorders characterized by an abnormality in the dystrophin gene). These diseases can lead to cardiac complications due to the progressive degeneration of skeletal and cardiac muscle fibers, which can result in myocardial inflammation and other cardiac abnormalities, including myocarditis. While the exact pathophysiological link remains fully elucidated, investigators have implicated the absence of dystrophin in the myocardial cells as the cause of spontaneous myocarditis. Specifically, the lack of dystrophin leads to increased susceptibility to injuries, inflammation, and necrosis, culminating in myocarditis. Similar mechanisms might apply to other forms of muscular dystrophy, like LGMD. Clinicians should realize that viral or “idiopathic” myocarditis should always be possible in any case, including the present one. Spontaneous myocarditis might be the mechanism by which the Q wave or old MI pattern we frequently see in muscular dystrophy patients with cardiomyopathy.
Consequently, all patients with LGMD should have a baseline cardiac evaluation, ECG, and structural heart studies with echocardiography or cardiac MRI to guide management. Definitive diagnosis often requires histological evidence typically gathered via endomyocardial biopsy, but it is rarely performed due to its invasive nature. A multidisciplinary team typically administers individualized management strategies depending on the severity of cardiac involvement and the subtype of LGMD, among other factors. Therapeutic measures include pharmacotherapy for HF and arrhythmias, cardio-protective lifestyle modifications, and, in severe cases, cardiac pacemaker or defibrillator implantation. Heart transplantation can be successful in some patients with LGMD R9 (FKRP-related) who developed severe HF. Gene replacement, editing, modulation, myoblast transplantation, and small molecule therapies are under investigation for treating various LGMD subtypes. Their effectiveness and safety remain to be determined. Lastly, some LGMD patients can also exhibit pulmonary complications (e.g., respiratory insufficiency, sleep disorder), swallowing or feeding problems leading to malnutrition, and increased risk for musculoskeletal spine deformities (e.g., kyphosis or scoliosis). All of these should be contained in the supportive care of patients with LGMD.
*A waddling or myopathic gait is a way of walking due to muscle weakness in the pelvic girdle. Besides Muscular dystrophy, a waddling gait may be associated with weakness in the hip girdle and upper thigh muscles, pregnancy, hemiplegia, severe osteoarthritis knee, sciatica, congenital hip dysplasia, etc.
**A peri-infarction block is part of an intraventricular conduction delay expressed as an initial or terminal slurring of the QRS complex that occurs after acute MI. It represents the formation of scar tissue with slow conduction. As a result, the QRS duration (QRSd) is prolonged (to >110 msec).
***Different types of muscular dystrophy:
Duchenne’s Muscular Dystrophy
Becker’s Muscular Dystrophy
Emery- Dreifuss Dystrophy
Facioscapulohumeral Dystrophy
Scapuloperoneal Syndrome
Oculopharyngeal Dystrophy
Congenital Muscular Dystrophies
Kearns-Sayre Syndrome
Myotonic Dystrophy
Limb-Girdle Muscular Dystrophies
Keywords:
muscular dystrophy, peri-infarction block, spontaneous myocarditis, spontaneous myositis
UpToDate:
Limb-girdle muscular dystrophy
Myocarditis: Causes and pathogenesis
Overview of and approach to the idiopathic inflammatory
myopathies

A 73-year-old woman, s/p ICD implanted for recurrent ventricular tachycardia (VT) associated with dilated cardiomyopathy (DCM) 9 months ago, was admitted after receiving several electrical
A 26-year-old Taiwanese man noted weakness in both lower legs and could not stand upon waking up. At the same time, he experienced upper back

This 30-year-old woman complained of having had about ten episodes of syncope associated with mild exertion for the past six months.
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung all right Reserved. Designed by 青澄設計 Greencle Design