Case 12
A 73-year-old woman, s/p ICD implanted for recurrent ventricular tachycardia (VT) associated with dilated cardiomyopathy (DCM) 9 months ago, was admitted after receiving several electrical
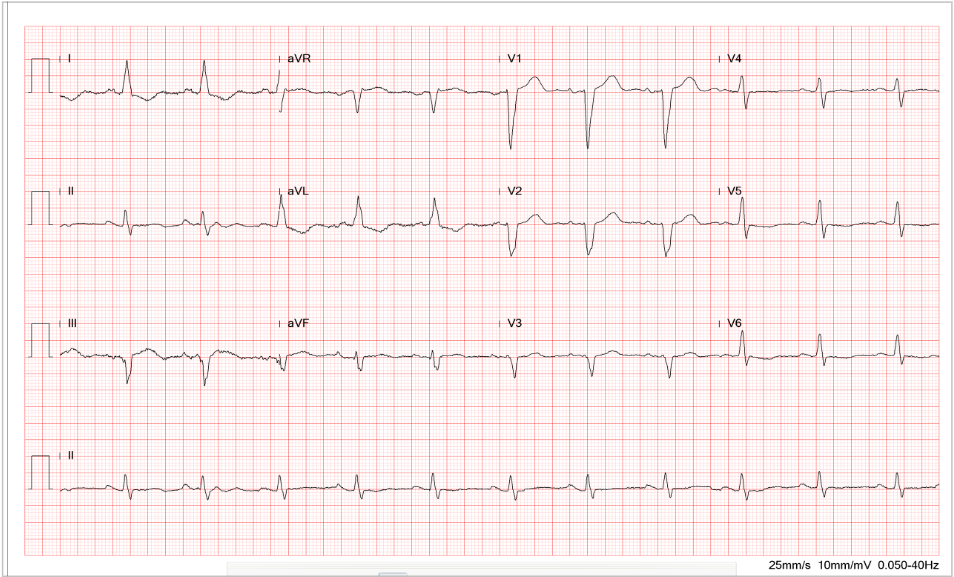
This 75-year-old man came to the Emergency Department (ED) complaining of abdominal pain for one day. He claimed he had constipation for several months, for which he took a laxative daily. He also noticed muscle weakness, especially in the lower extremities, which he accounted for his falls while climbing a staircase and riding a bike within the past five days. When seen, he appeared chronically ill with abdominal pain; blood pressure measured 194/114 mmHg, which became 146/95 mmHg after the easing of pain. BT/PR/RR was 36.6 ºC, 98/min, 18/min. The heart and lungs were unremarkable. The abdomen was soft and not tender; the bowel sounds were barely audible (hypoactive). The Murphy sign was negative. There was no apparent muscular atrophy, but notably, there was a decrease in muscle power with reduced deep tendon reflexes in both legs. ECG showed sinus rhythm at 95/min with borderline first-degree AV block (P-R interval 200 msec) and Q waves in leads III and AVF, suggesting an old interior MI. There were diffuse flattening T waves with prolonged QT interval (QTc >455ms)*. Pertinent laboratory data included Hb 11.2 gm/dL, Hct 33%, WBC 6710, Na 132 mEq/L, K 2.0 mEq/L, Cl 100 mEq/L Ca 8.6 Mg 2.0, BUN 30 mg%, Cr 1.2 mg%, CK 40 [N 38~397] U/l, ESR-1hr 10 [N 0~10] mm/hr, TSH 0.349 [N 0.27~4.2] uIU/ml, free T4 1.510 [N 0.93~1.71] ng/dl, and serum osmolarity 274 (N 275 – 295) mOsmol/Kg. The patient declined blood drawing for arterial blood gas (ABG) analysis. The peripheral venous blood gas (PVBG)** showed pH 7.512, pCO2 44.4 mmHg, pO2 35 mmHg), O2sat 72.8 (%), and HCO3– 34.8 mEq/L, indicative of metabolic alkalosis. 24-hour urine (3420 ml) analysis showed urine osmolarity378 (N 500 to 850) mOsmol/Kg with Cr 27.7 (N 30~125) mmol/L, K 19.3 (N12~62) mmol/L), and Na 88 (N 20~110) (mmol/L), and ammonia 40 (N 30 mmol/L). Given significant hypokalemia and metabolic alkalosis, the care team started potassium supplement with 0.9% saline containing 40 mEq K/L infusion at 10 mEq K/hr. Subsequent gastrointestinal (GI) work-up was negative for any infection, and his abdominal pain gradually resolved. In consultation with the renal specialist, the cause of his abdominal pain, polyuria, hypertension, and lower extremity weakness was primarily due to chronic potassium loss and insufficient potassium intake***, both of which, in combination, resulted in hypokalemia with metabolic alkalosis. The assumption was suggested by the patient’s having been taking only cereals and constantly using laxatives for the past several months. After potassium supplements, he gradually improved and could return home in 10 days.

Sinus rhythm at 95/min.
Borderline Ist degree AV block (PR interval 0.20s)
QT interval prolongation (QTc >455ms)
Q waves in III and AVF suggestive of an old interior wall MI
Nonspecific ST-T wave changes (flattened T fused with P)
Unstable baseline
The finding of low serum potassium (K 2.0 mEq/L) is significant. The average serum potassium level ranges from 3.5 to 5.0 mEq/L. Clinically, hypokalemia is defined as a serum potassium level <3.5 mEq/L. Moderate hypokalemia is defined as serum potassium levels of 2.5 to 3 mEq/L, and severe hypokalemia as a serum potassium level <2.5 mEq/L, like in the present case. The diffuse flattening of the T wave, borderline first-degree A-V block (P-R interval 200 msec), and QT prolongation (QTc >455 msec) are in line with hypokalemia found clinically. Other ECG features caused by hypokalemia**** (K < 2.7 mEq/L) may include ST-segment depression, T-wave inversion, and prominent U waves (best seen in the precordial leads V2-V3). When hypokalemia is associated with hyperthyroidism, sinus tachycardia, A-V block, and increased QRS voltage may be additionally observed. With resultant QT interval prolongation, there is a potential to develop life-threatening ventricular tachyarrhythmias (e.g., VT, Torsades de Pointes, and VF) leading to sudden death.
Potassium is the principal intracellular ion of most body tissues. It is involved in several essential processes, including regulating the heartbeat, ensuring proper function of the muscles and nerves, and maintaining normal renal function. Enteral potassium is usually well absorbed from the GI tract and is predominantly excreted by the kidneys. The ratio of intracellular to extracellular potassium determines, in part, the cellular membrane potential. Thus, small changes in the extracellular potassium level can significantly affect the function of the cardiovascular and neuromuscular systems. Clinically, mild hypokalemia rarely causes symptoms. Moderate hypokalemia can cause muscle weakness and may lead to paralysis and respiratory failure. Other muscular dysfunction includes cramping, fasciculations, paralytic ileus, hypoventilation, hypotension, tetany, and rhabdomyolysis. Lastly, longstanding hypokalemia can cause metabolic alkalosis and impair renal concentrating ability (hypokalemic nephropathy) *****, causing polyuria with secondary polydipsia (nephrogenic diabetes insipidus). Furthermore, associated hypovolemia due to vomiting or diarrhea can activate the renin-angiotensin-aldosterone system (RAAS) system, thereby causing hypertension, as illustrated in the present case.
Patients with muscle weakness or paralysis are not infrequently encountered in ED. The causes of muscle weakness and paralysis can be neuromuscular (e.g., Guillain-Barré syndrome, myasthenia gravis, transverse myelitis, autoimmune myositis, etc.), metabolic, and psychological disorders. Among the metabolic disorders, hypokalemia is the most common. The causes of hypokalemia are multiple. While a decrease in potassium intake is rare, excessive loss of potassium from the GI tract or urine is more likely to be the cause. Abnormal GI potassium losses occur in chronic diarrhea, including regular use of laxatives, like in the present case, and bowel diversion, clay (bentonite) ingestion (binding potassium and decreasing its absorption), and, rarely, villous adenoma of the colon (massive potassium secretion). Clinically, volume depletion caused by vomiting or gastric suction can lead to metabolic alkalosis and enhanced aldosterone secretion; aldosterone and metabolic alkalosis foster the kidneys to excrete more potassium.
The transcellular shift of potassium into cells, a distinctly different mechanism, may also cause hypokalemia. This shift can occur in any situation like insulin injection, familial periodic paralysis, ****** glycogenesis during total parenteral nutrition or enteral hyperalimentation (stimulating insulin release), excessive activation of the sympathetic nervous system like the use of beta 2-agonists (albuterol, terbutaline, etc.), and during hyperthyroidism (hypokalemic periodic paralysis}.
Various disorders can manifest hypokalemia with enhanced renal potassium excretion due to excess mineralocorticoid (i.e., aldosterone). These disorders include adrenal steroid excesses like Cushing syndrome, primary hyperaldosteronism, renin-secreting tumors, glucocorticoid-remediable aldosteronism, and congenital adrenal hyperplasia, excessive production of renin and aldosterone (e.g., Bartter syndrome, Gitelman syndrome). Additionally, ingestion of substances such as licorice containing glycyrrhizin, which inhibits the enzyme 11 beta-hydroxysteroid dehydrogenases (11β-HSDH), preventing the conversion of cortisol (some mineralocorticoid activity), resulting in renal potassium wasting. Diseases that can cause renal potassium wasting also include Liddle syndrome, congenital and acquired renal tubular disorders, such as renal tubular acidosis, and Fanconi syndrome.
Clinically, many medications can cause hypokalemia. Among them, diuretics, i.e., loop diuretics, osmotic diuretics, and thiazide diuretics, are by far the most commonly encountered. By inducing diarrhea, laxatives, when used regularly, can cause hypokalemia. Other remedies include amphotericin B, antipseudomonal penicillin (e.g., carbenicillin), penicillin in high doses, and theophylline. Hypomagnesemia often shares the exact cause as hypokalemia (diarrhea and use of laxatives), and it often accompanies hypokalemia, and it may also result in increased renal potassium losses.
Although K+ supplements can facilitate muscle recovery and reduce the risk of cardiac arrhythmia, it is essential to note that intravenous potassium supplements should never be given by direct IV injection, subcutaneously, or intramuscularly. Moreover, the dosage should be minimized to avoid rebound hyperkalemia on recovery, especially in patients with hypokalemia caused by acute K+ shift into cells. Clinicians also need to know that the rapidity and method of potassium repletion depends on the severity of hypokalemia, the presence of associated conditions, and the presence or absence of signs and symptoms. The following guidelines are helpful:
5. Electrolytes should be closely monitored to avoid rebound hyperkalemia and to determine the necessity of continuing potassium supplements.
*Normal QT interval: The QT interval varies with heart rate. Females have a longer QT interval because they have less IKs potassium channel. After correction for heart rate, normal QTc intervals are <450 msec for men and <460 msec for women. A QTc between these values and 500 msec is considered prolonged. When the QT interval is prolonged, there is likely the presence of dispersion of refractoriness, i.e., a considerable variation in refractory periods across different heart regions. Consequently, it favors the development of unidirectional block and reentry-arrhythmogenesis of reentrant arrhythmias.
** The typical ranges of each parameter in venous blood gas analysis are pH: 7.32 to 7.42, pCO2: 38 to 52 mmHg, HCO3– 22 to 28 mEq/L, and SaO2: 60% to 80%. There is little difference between the pH obtained from the PVBG and the ABG, with the arterial pH typically 0.03 higher than the venous pH (95% confidence interval 0.029–0.038). However, pO2 and pCO2 vary significantly between arterial and venous samples. The arterial pO2 is typically 36.9 mm Hg greater than the venous with significant variability (95% confidence interval from 27.2 to 46.6 mm Hg). Venous and arterial pCO2 were not comparable either because the 95% prediction interval of the bias for venous pCO2 was unacceptably wide, extending from −10.7 mm Hg to +2.4 mm Hg. Clinically, only the pH of venous blood gas analysis is applicable.
***Daily potassium intake for a healthy adult is between 2,300 mg and 3,400 mg, and WHO recommends consuming at least 3,510 mg of potassium daily. However, in patients with renal dysfunction, the amount of potassium intake should be reduced for fear of the development of hyperkalemia. Potassium can be found in various foods, such as bananas, beets, broccoli, potatoes, tomatoes, spinach, cauliflower, etc.
***Daily potassium intake for a healthy adult is between 2,300 mg and 3,400 mg, and WHO recommends consuming at least 3,510 mg of potassium daily. However, in patients with renal dysfunction, the amount of potassium intake should be reduced for fear of the development of hyperkalemia. Potassium can be found in various foods, such as bananas, beets, broccoli, potatoes, tomatoes, spinach, cauliflower, etc.
****In contrast, during hyperkalemia, the T wave is “pulled upwards,” creating tall “tented” T waves and stretching the remainder of the ECG to cause the P wave to flatten and QRS to widen. However, the P-R interval can also be prolonged during hyperkalemia.
***** Persistent hypokalemia can impair urinary concentrating ability, cause intracellular acidosis (by inducing K+ to exit from cells, which is counterbalanced by a movement of H+ into cells [H+/K+ pump]), increase ammonia production, increase bicarbonate reabsorption (metabolic alkalosis), and alter sodium reabsorption. Ultimately, it can result in tubulointerstitial injury and fibrosis, referred to as hypokalemic nephropathy. Hypokalemic nephropathy might be reversible within one month after correction of hypokalemia
******Familial periodic paralysis is an autosomal dominant disorder characterized by transient episodes of profound hypokalemia caused by sudden abnormal potassium shifts into cells. Typically, a large carbohydrate meal or strenuous exercise triggers the attack with varying degrees of paralysis.
Keywords:
hypokalemia, hypokalemic nephropathy, periodic paralysis, renin-angiotensin-aldosterone system (RAAS)
UpToDate:
Causes of hypokalemia in adults
Evaluation of the adult patient with
hypokalemia
Clinical manifestations and treatment of
hypokalemia in adults
Hypokalemia-induced kidney dysfunction

A 73-year-old woman, s/p ICD implanted for recurrent ventricular tachycardia (VT) associated with dilated cardiomyopathy (DCM) 9 months ago, was admitted after receiving several electrical
A 56-year-old man came to the Emergency Department because of worsening dyspnea with a productive cough for the past two weeks. Notably, he smoked one
This 50-year-old man came to the Emergency Department complaining of having substernal chest pain for more than 10 hours. The pain was sharp and not
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung all right Reserved. Designed by 青澄設計 Greencle Design