Case 33
This 35-year-old male was transferred from a local hospital for further evaluation after a biopsy of a hard, 4 cm in diameter lymph node in
A 67-year-old woman experienced progressive dyspnea with PND and leg edema for two weeks. PMH was significant for chronic hypertension for 20 years.
Two years prior, she also had endometrial carcinoma and received a 5-month course of chemotherapy (doxorubicin 335.85mg and cisplatin 310.25mg in total) followed by irradiation therapy for metastatic neck lymphadenopathy.
Of note, Echocardiography documented EF of 69.9% and 69.1% associated with hypertensive cardiomyopathy, respectively, before and after the completion of chemotherapy. She had biventricular heart failure on admission (BT: 37.3 ℃, PR:108/min, RR:18/min, BP:115/67mmHg, JVD 11 cm, PMI displace to the left anterior axillary line, Gr2/6 systolic murmur at the apex, and left sternal border, bilateral basilar rales, and leg edema).
CBC and Cardiac enzymes were normal, as were electrolytes and liver enzymes. BNP measured 749 (N:<100) pg/mL, and BUN 31 mg/dL CR 0.8 mg/dL. Echocardiography revealed a dilated heart with EF of 21.6%, severe TR, and mild MR. There was no evidence of acute myocardial ischemia/infarction or myocarditis, and the presumptive diagnosis was the worsening of heart failure due to anthracycline-induced cardiomyopathy.
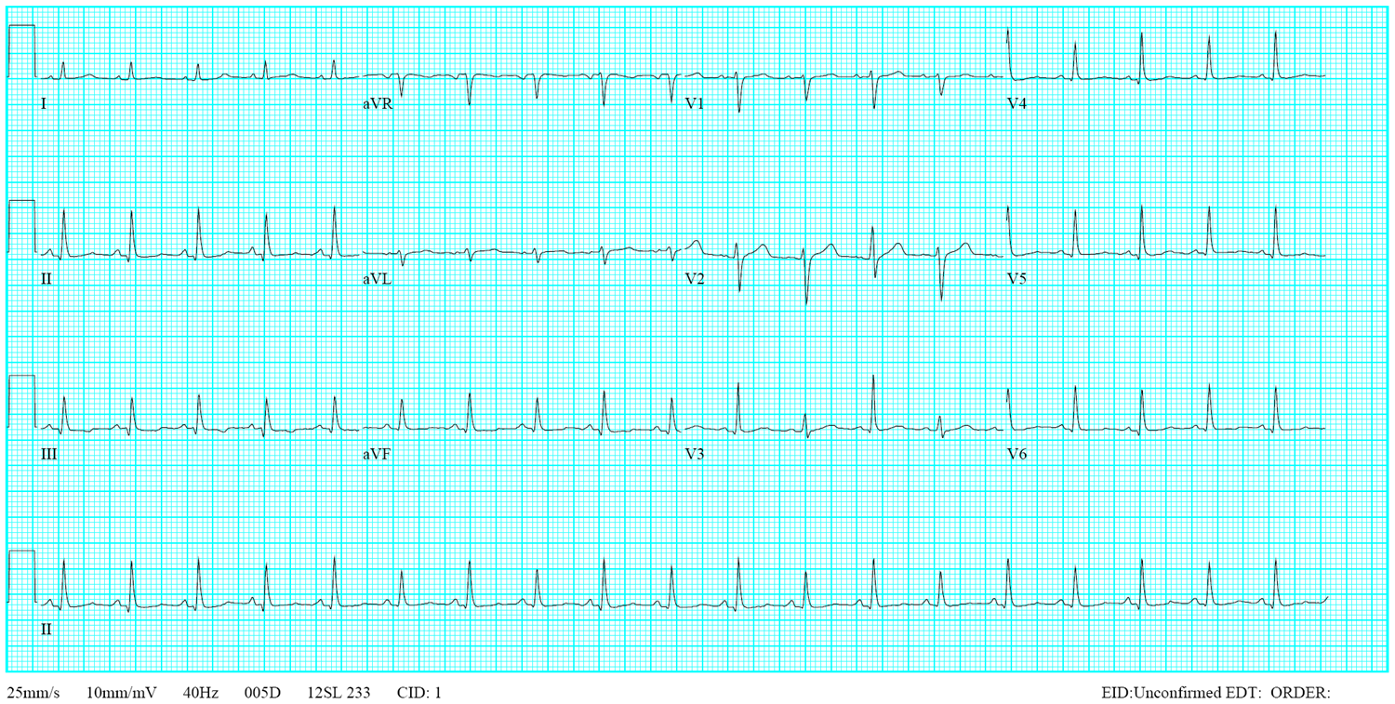
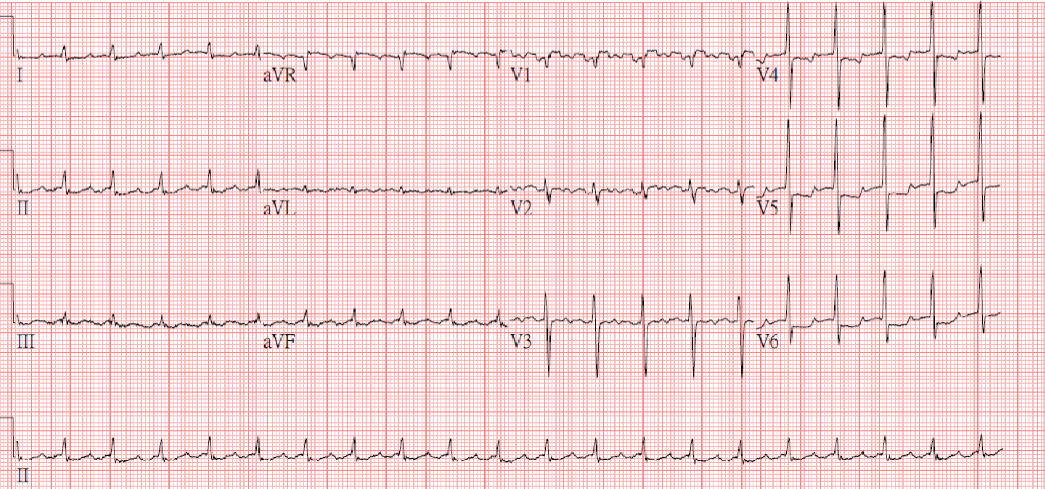
Sinus tachycardia at 105/min.
Low voltage at limb leads
LVH and LA enlargement
Tiny r waves in V2-V4, r/o old anteroseptal MI
QT interval prolongation (QTc: 523ms) with nonspecific ST-T changes
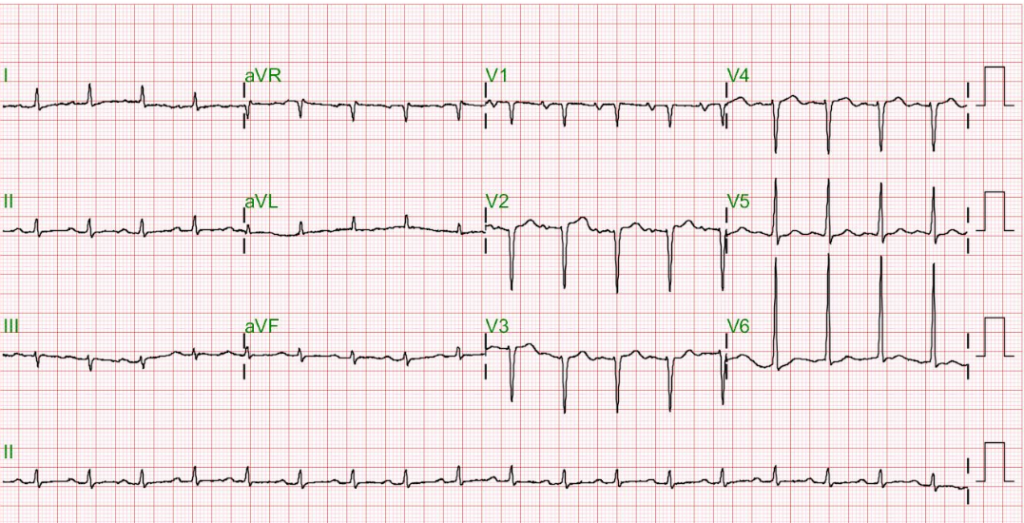
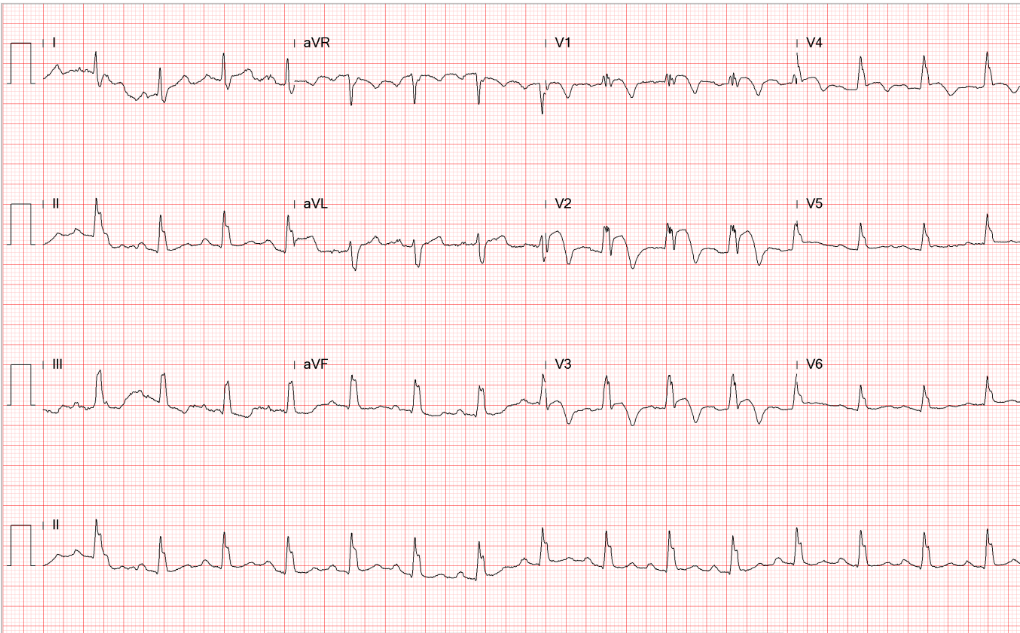
Sinus rhythm at 80 /min
LVH by voltage criteria
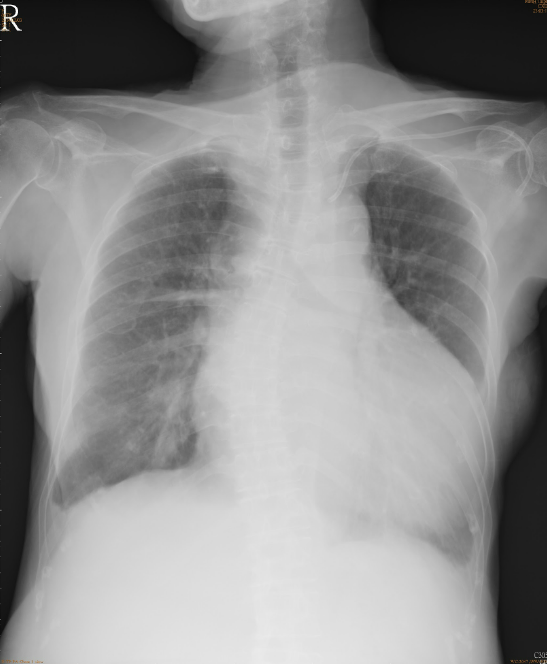
A marked increase in the cardiothoracic ratio due to cardiomegaly, r/o pericardial effusion.
Pulmonary congestion with bilateral pleural effusion.
LA enlargement
An increase in lung volume suggestive of COPD
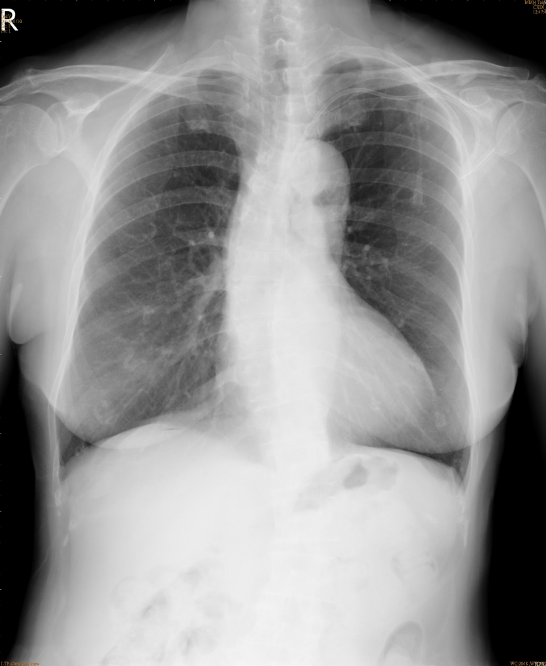
Tortuous aorta
LV enlargement
An increase in lung volume suggestive of COPD
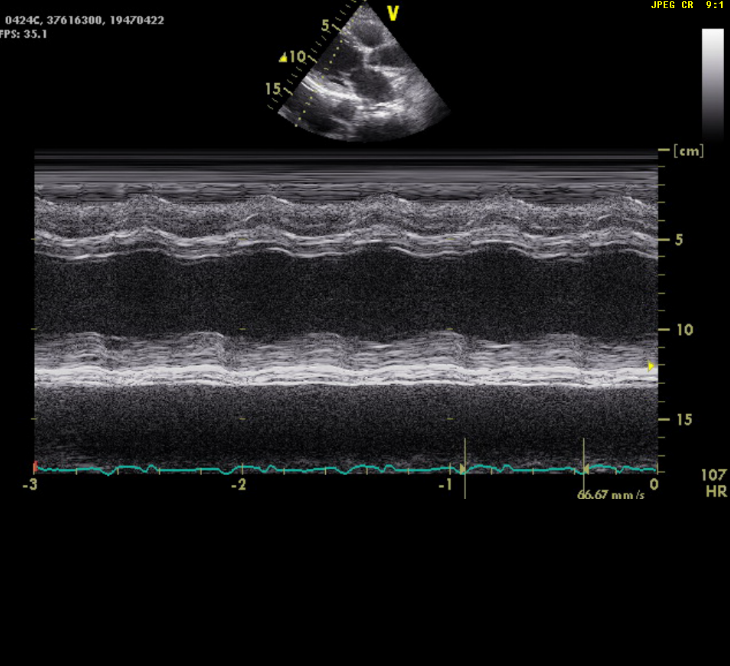
Dilated heart (enlargement of all chambers) with EF of 21.6%
Severe TR and mild MR (not shown)
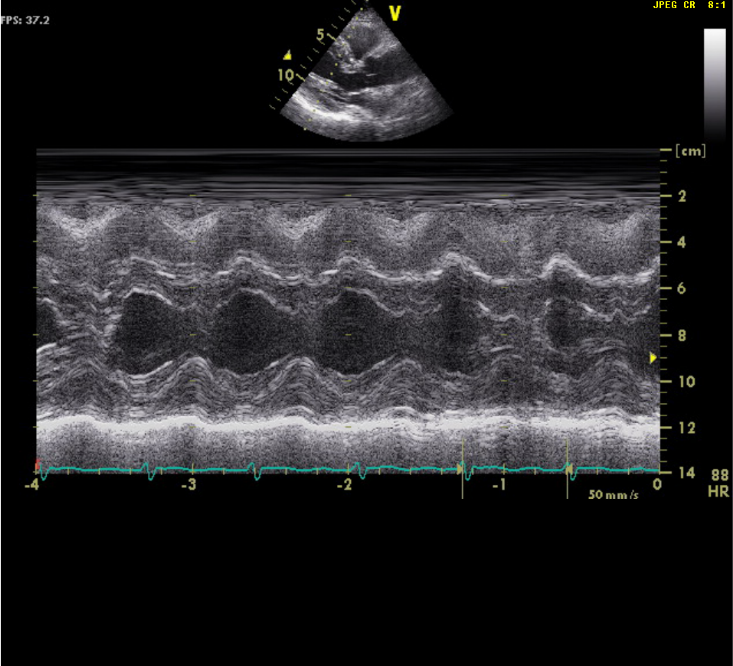
LV concentric hypertrophy
EF of 69.9%
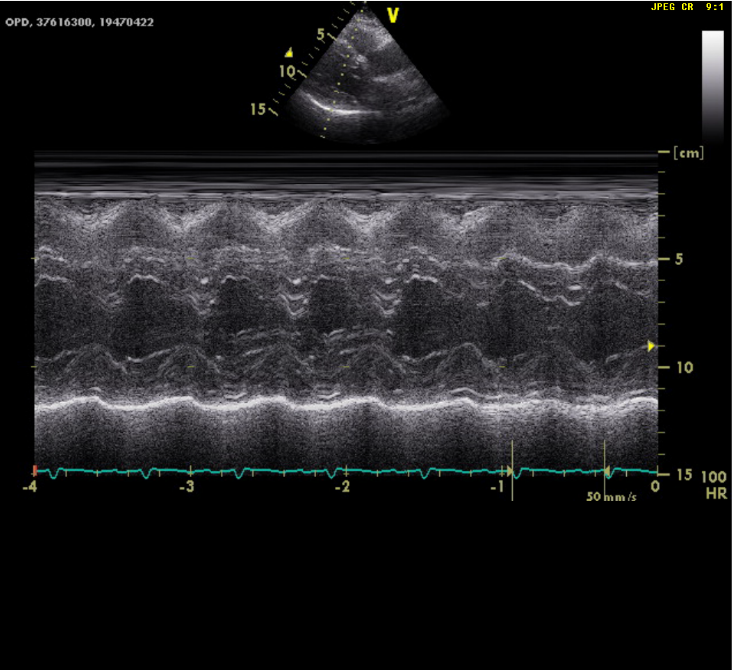
LV concentric hypertrophy
EF of 69.1%
The conversion of hypertensive cardiomyopathy to anthracycline-induced dilated cardiomyopathy, as shown by changes in the chest X-ray and echocardiography, is reflected by the reduction of the QRS voltage in limb leads and loss of r waves in V2-V4 on ECG. However, differential diagnoses include atherosclerotic coronary heart disease with prior MI (coronary angiography) and dilated cardiomyopathy caused by previous myocarditis (viral serology).
Doxorubicin, an anthracycline antibiotic, can cause defective mitochondrial biogenesis (iron accumulation), increase reactive oxygen species, induction of apoptosis, deoxyribonucleic acid (DNA) damage through interaction with topoisomerase II, inhibition of protein synthesis, and dysregulation of cardiomyocyte autophagy resulting in myofibrillar disarray and vacuolization of cardiomyocytes. Doxorubicin cardiotoxicity is cumulative and typically occurs at an average total dose of 500 mg/m2; however, studies have shown that cardiotoxicity can arise in a cumulative amount of as low as 300 mg/m2.
Clinically, the cardiotoxic effects of doxorubicin can be acute, sub-acute, and even chronic (more than one year and even ten years late). The resultant ECG changes often exhibit low QRS voltage with left axis deviation and nonspecific ST-T changes. Besides HF, sudden cardiac death has been associated with anthracycline use and reported as late as 15 years after therapy. The cardiotoxic effect of doxorubicin is continuous upon exposure. Prompt identification of cardiotoxicity is possible by periodic serum troponin measurement and echocardiographic LV EF assessment. Doxorubicin cardiotoxicity reversibility occurs in almost two-thirds of cases. Early detection of chemotherapy-induced cardiotoxicity followed by appropriate treatment (e.g., angiotensin-converting enzyme inhibitors, mainly enalapril, and beta-blockers like carvedilol or bisoprolol) is crucial to prevent irreversible cardiac dysfunction.
Lastly, radiation therapy leads to the formation of free radicals, which can cause molecular damage, leading to cardiac fibrosis (replacement of the interstitium and dead myocytes by collagen and fibrotic tissue), thereby increasing the risk of coronary artery disease (initially, endothelial injury), cardiomyopathy (mainly restrictive cardiomyopathy with diastolic dysfunction), valvulopathy, arrhythmias, and pericardial disease. However, it usually occurs in patients receiving irradiation to the mediastinum for breast and lung cancers, lymphoma, etc.
Keywords:
anthracycline-induced dilated cardiomyopathy, doxorubicin, radiation therapy, restrictive cardiomyopathy
UpToDate:
Clinical manifestations, diagnosis, and treatment of anthracycline-induced
Cardiotoxicity: Risk and prevention of anthracycline cardiotoxicity
Cardinale D et al. Cardiotoxicity of anthracyclines. 2020, Frontier Cardiovascular Medicine
March 18. doi: 10.3389/fcvm.2020.0002
Belzile-Dugas E and Eisenberg MJ. Radiation‐induced cardiovascular disease: Review of an underrecognized pathology. Journal of the American Heart Association. 2021;10:e021686

This 35-year-old male was transferred from a local hospital for further evaluation after a biopsy of a hard, 4 cm in diameter lymph node in
A 43-year-old woman, s/p mitral valve (MV) replacement (Bjork-Sheily) for mitral stenosis (MS) associated with rheumatic heart disease 18 years ago, was admitted with worsening
This 43-year-old woman was rushed to ER because of the sudden onset of substernal chest tightness accompanied by cold sweats, which lasted more than 30
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.