Case 12
A 73-year-old woman, s/p ICD implanted for recurrent ventricular tachycardia (VT) associated with dilated cardiomyopathy (DCM) 9 months ago, was admitted after receiving several electrical
A 30-year-old woman, a professional cook, suddenly experienced retrosternal chest tightness after dinner, which increased in intensity without radiation, lasting for 2 hours. She also described the associated development of dyspnea, cold sweating, and nausea/vomiting. PMH was only significant for smoking cigarettes 2 PPD for over ten years. There was no known history of diabetes mellitus or hypertension, and the family history was unremarkable. At Emergency Department, she appeared acutely ill and in moderate respiratory distress. The skin was cold but with no cyanosis. Under sedation, BT measured 36.4 ℃, BP 106/73 mmHg, PR 140/min (regular), and RR 16/min. Chest X-ray showed a normal-sized heart and prominent pulmonary artery (PA) with increased pulmonary vascular markings suggesting an early stage of heart failure (HF). Notably, ECG showed ST elevation in V2-V5, II, III, and aVF suggestive of acute MI, and there was marked elevation of cardiac enzymes: CKMB 268.7 (N <5.0) ng/mL, and troponin I 192.4 (N <0.5) ng/mL. Other laboratory data included Hb 13.6 g/dL, WBC 21,000 /uL, BUN 9 mg/dL, Cr 0.9 mg/dL, and normal electrolytes. Accordingly, emergency cardiac catheterization was in order. However, she had a cardiac arrest due to ventricular fibrillation (VF) and developed cardiogenic shock with florid pulmonary edema before coronary angiography. ABG obtained at cardiac arrest showed pH 7.06 (N 7.35-7.45), pCO2 13 (N 32-45) mmHg, pO2 222 (N 75-100) mmHg (on O2 supply), HCO3 3.7 (N 20-26) mmol/L, BE -24 (N -2.0 – +2.0) mmol/L; accordingly, she then had an ECMO instituted, followed by an IABP insertion. Surprisingly, subsequent coronary angiography revealed a proximal LAD aneurysm with thrombus formation preceded by a left main coronary artery (LMC) stenosis (50%). Echocardiograms showed no chamber enlargement, but LV was hypokinetic with a reduced EF (30%). The care team then contemplated a surgical consultation.

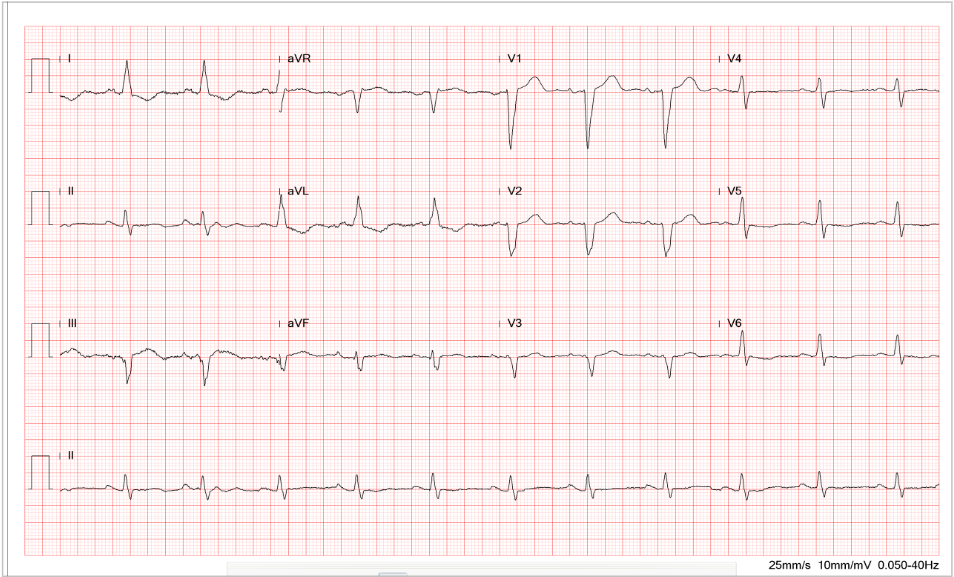
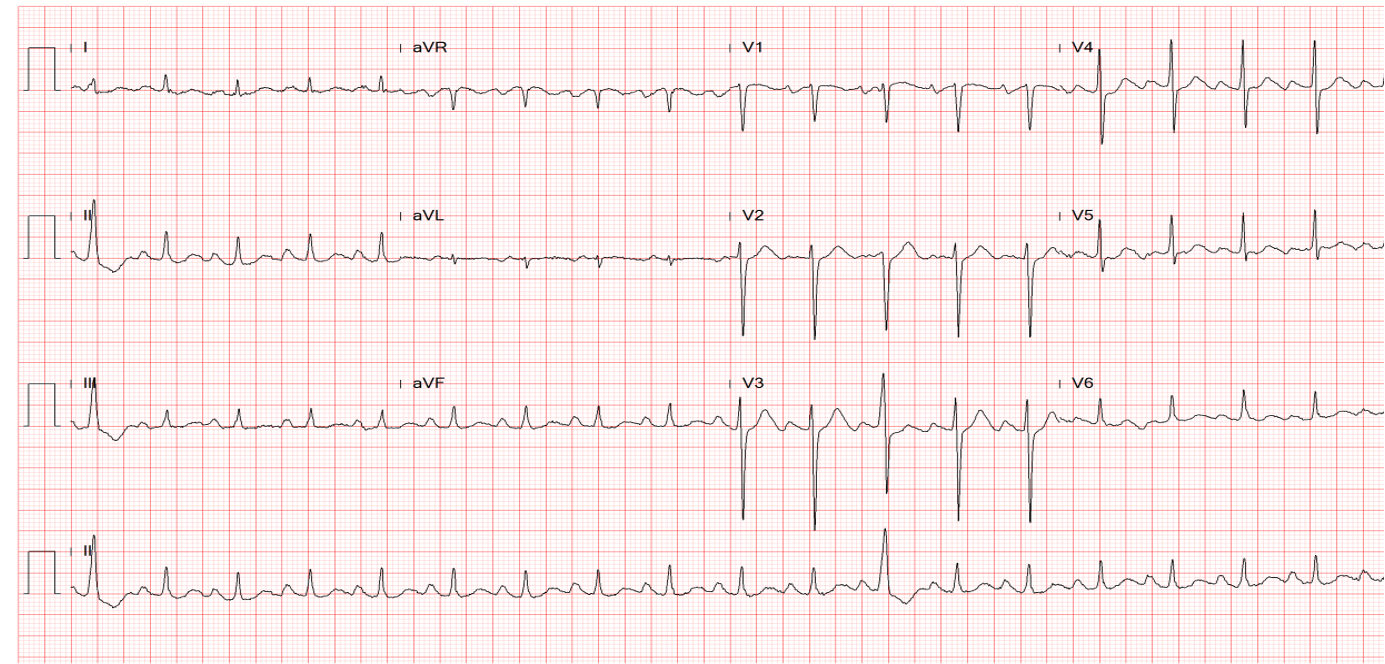
Sinus tachycardia at 145/min
Indeterminate QRS axis (-60°) with ST-segment elevation and alternans, along with alternating complete and incomplete RBBB patterns (QRS alternans) best seen in leads I, II, aVR, and V1-V6.
These findings suggest extensive acute anterolateral MI with a propensity to ventricular fibrillation (VF).
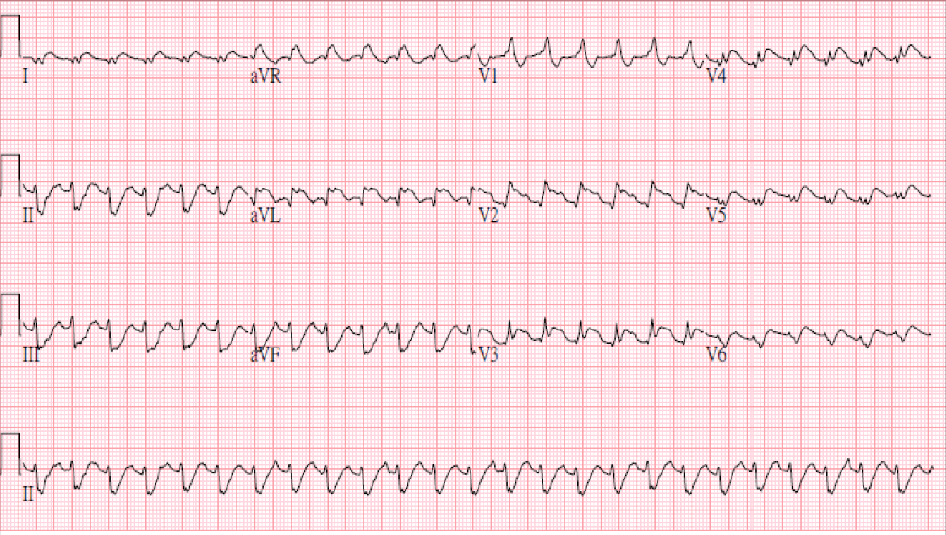
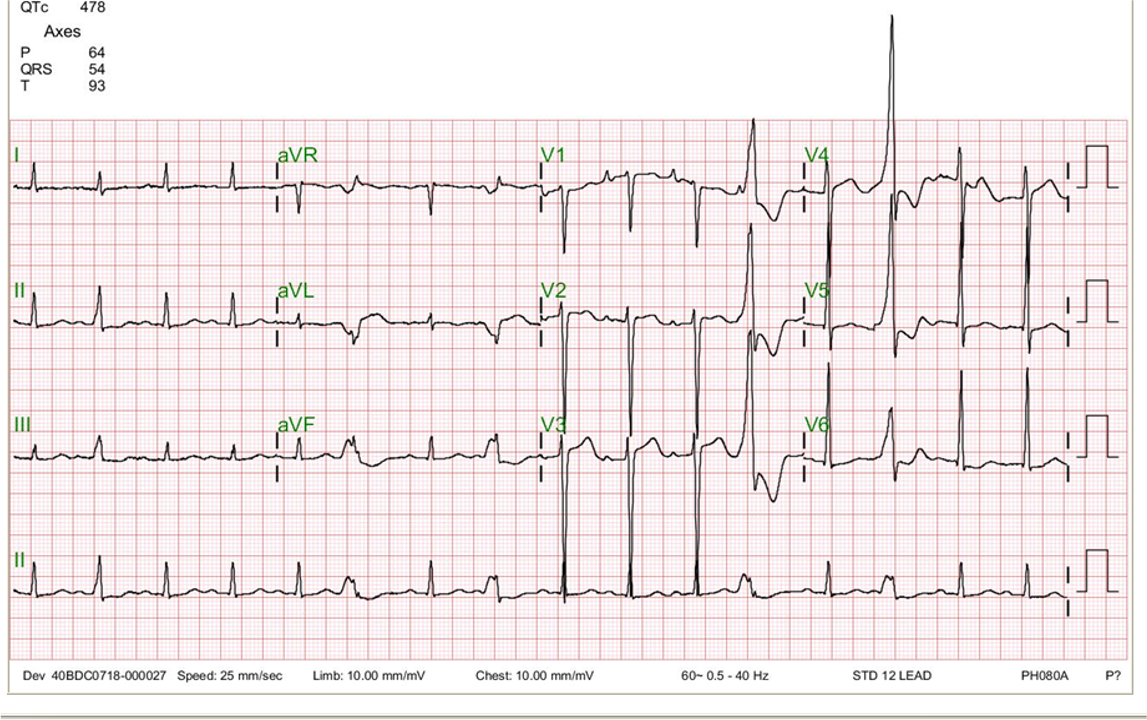
Sinus tachycardia at 150/min; Superior axis (> -90°) with a complete RBBB pattern (bifascicular block)
Marked ST elevation in leads I, aVL, and V2-V6 suggestive of extensive anterolateral MI.
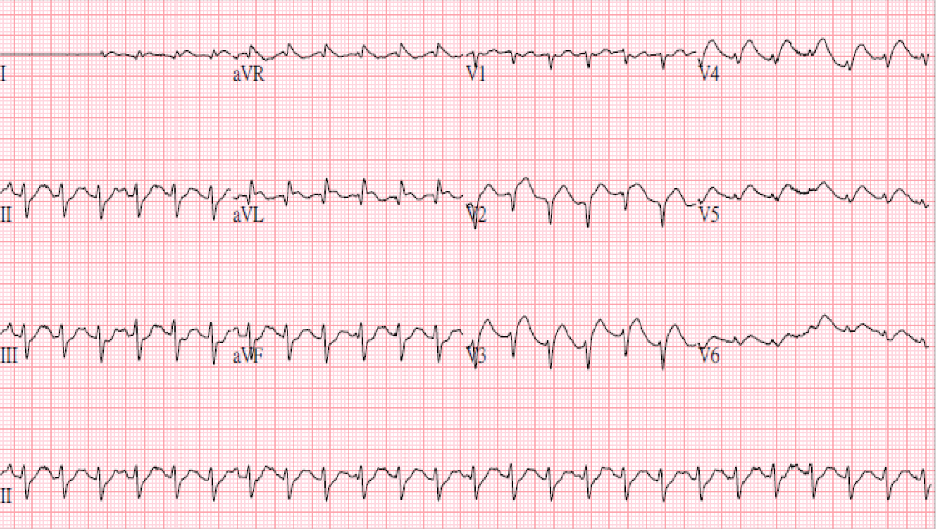
Sinus tachycardia at 152/min; superior QRS axis; Q waves in V1-V2; ST elevation in
Leads I aVL, V2-V6 suggestive of extensive MI
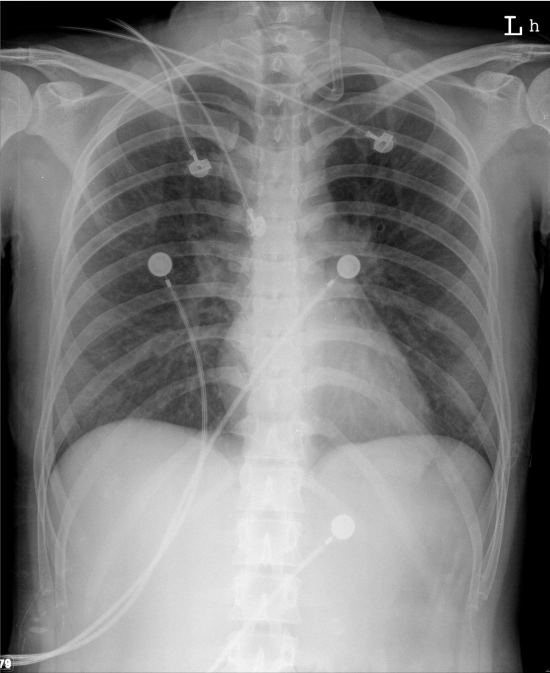
Increased pulmonary vascular markings associated with prominent (bilateral) PA suggestive of the early stage of HF
Normal cardiac size
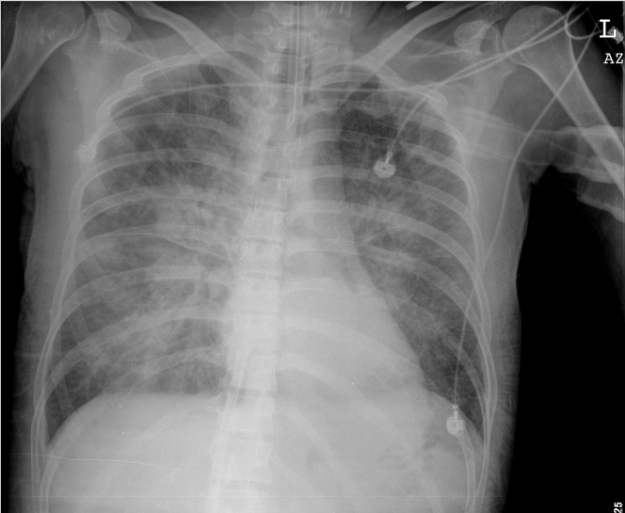
Florid pulmonary edema in the absence of cardiomegaly after cardiac arrest
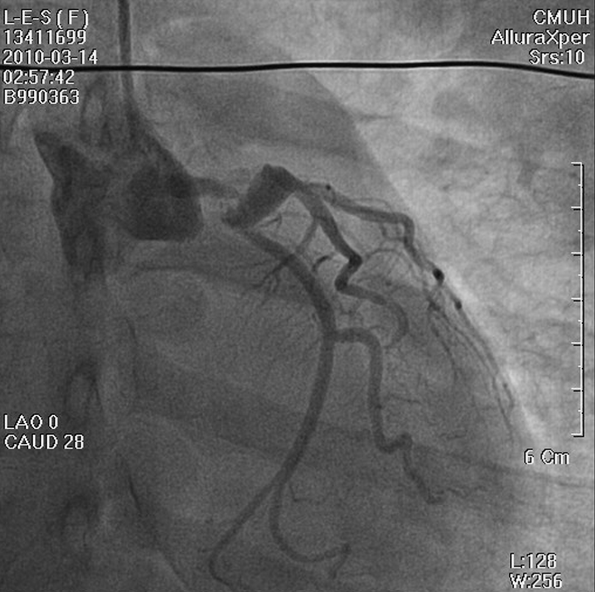
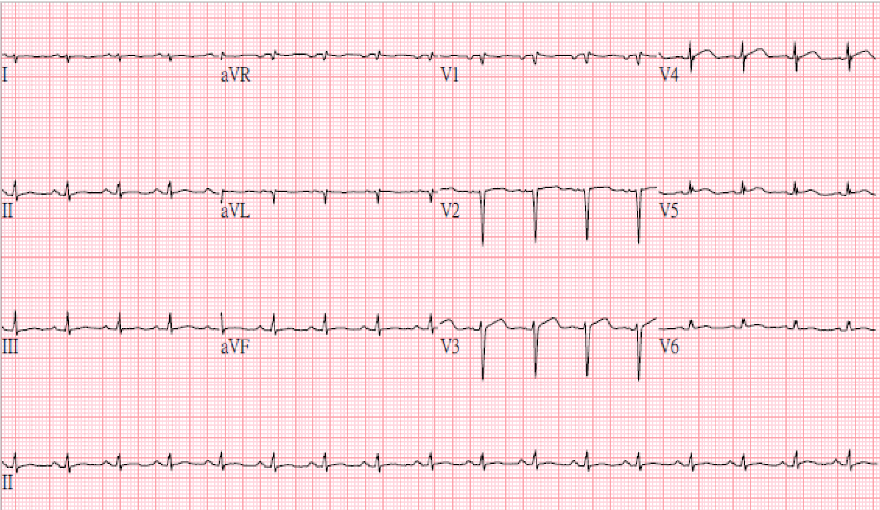
LMC stenosis (50%) preceding a proximal LAD aneurysm (white arrow) with thrombus formation
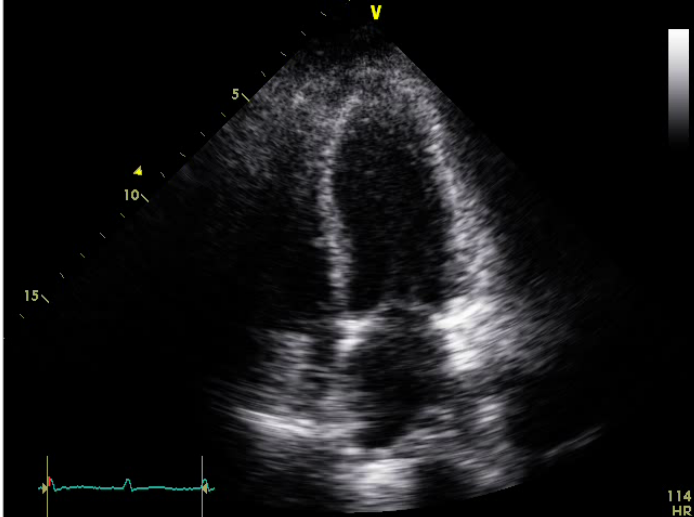
No chamber enlargement
Hypokinetic LV with a reduced EF (30%)
The most striking ECG finding on admission is ST-T and QRS alternans alongside ST elevation in leads I, aVL, and V1-V6 during sinus tachycardia at 145/min. The elevation of the ST segment indicates an acute phase of myocardial infarction (injury), and ST-T and QRS alternans signal spatial discordant ventricular repolarization. The ST-T and QRS alternans are among the forms of “cardiac alternans.”* The underlying pathophysiological mechanism responsible for the ST-T and QRS alternans is complex, as it involves cellular restitution of action potential changes due to altered intracellular calcium cycling. Nevertheless, clinical etiologies commonly include acute myocardial ischemia or MI, digitalis intoxication, long QT syndromes, and catecholaminergic polymorphic ventricular tachycardia (CPVT). The ST-T (T wave) alternans with or without QRS alternans suggest marked dispersion of repolarization, which creates a tissue substrate highly vulnerable to reentry, a propensity to malignant ventricular tachyarrhythmias (VT, VF) leading to cardiac arrest. Indeed, the patient had a cardiac arrest due to VF and was in cardiogenic shock requiring ECMO and IABP during coronary angiography.
Secondly, the development of bifascicular block (left AD and RBBB) in acute MI indicates the occlusive lesion is located at proximal LAD or LMC, which might cause extensive MI complicated by HF and later ventricular aneurysm formation.
Thirdly, an MI occurring at this young age (30 years old), premature atherosclerotic CHD should be first suspected. The coronary artery aneurysm (CAA) with thrombus formation unveiled by coronary angiography is unexpected and is the culprit of acute MI. Whether the patient contracted Kawasaki disease during childhood, which led to the formation of the CAA, can only be speculative.
CAA is a focal segmental dilation at least 1.5 times the adjacent normal segment. The term “coronary artery ectasia” describes similar but more diffuse lesions. Morphologically, a CAA can be saccular or fusiform. There are diverse etiologies for CAA, the most common being atherosclerotic coronary heart disease (usually male with 3-vessel disease). However, it can be idiopathic or congenital (genetic susceptibility), systemic vasculitis (e.g., Kawasaki, Takayasu, lupus erythematosus, periarteritis nodosa, Marfan syndrome), drug-induced (e.g., cocaine, amphetamines), infectious (bacterial, mycobacterial, fungal, syphilitic, Lyme, and septic emboli), and traumatic or iatrogenic (e.g., post-balloon angioplasty, stenting, atherectomy, and CABG graft aneurysm). With thrombus formation, a patient with a CAA may be asymptomatic, develop exertional symptoms such as angina, MI, and SCD, or manifest an acute coronary syndrome due to in-situ thrombus, distal embolization, or aneurysm rupture. Of note, a paradoxical worsening of ischemia after using nitroglycerin via a mechanism termed “dilated coronaropathy” may occur in patients with CAA. The CAA treatment may include surgical (e.g., CABG, aneurysm ligation, resection, or marsupialization with interposition graft), percutaneous (e.g., covered stents), and medical interventions.
*Cardiac alternans can be electrical or mechanical. Both can occur either alone or in combination. “Mechanical alternans” is called “pulsus alternans,” characterized by beat-to-beat variability of the arterial pressure waveform as identified on physical examination and echocardiograms as might be seen in patients with severe cardiac dysfunction such as severe aortic stenosis. “Electrical alternans” is the beat-to-beat variability of the QRS complex on the electrocardiogram. In the latter, simply the QRS amplitude alternans is seen in nonspecific tachycardia or pericardial effusion due to “swinging of the heart. On the other hand, if it involves the QT amplitude and morphology, referred to as “T wave alternans” seen in patients with acute myocardial ischemia, digitalis toxicity, CPVT, and long QT syndromes, it is an electrophysiological phenomenon associated with impending ventricular tachyarrhythmias prone to sudden cardiac death as shown in the present case.
Keywords:
coronary artery aneurysm (CAA), dispersion of repolarization, electrical alternans, intracellular calcium cycling
UpToDate:
Cardiovascular sequelae of Kawasaki disease: Management and prognosis
Qu Z and Weiss JN. Cardiac alternans: From bedside to bench and back. Circulation Research 2023;132, 127-149
Cohen P, O’Gara PT. Coronary artery aneurysms: A review of the natural history, pathophysiology and management. Cardiology in Review, 2008;16:301-304
Kawsara et al. Management of coronary artery aneurysms. JACC 2018;11:1211-1223

A 73-year-old woman, s/p ICD implanted for recurrent ventricular tachycardia (VT) associated with dilated cardiomyopathy (DCM) 9 months ago, was admitted after receiving several electrical
This 72-year-old man, a retired coal miner with a known history of chronic obstructive pulmonary disease (COPD) and pneumoconiosis, came to the Emergency Department complaining
This 92-year-old man complained of progressive dyspnea with PND, productive cough with whitish sputum, and bilateral leg edema for the past few weeks. He was
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.