Case 16
This 43-year-old woman was rushed to ER because of the sudden onset of substernal chest tightness accompanied by cold sweats, which lasted more than 30
A 43-year-old woman, s/p mitral valve (MV) replacement (Bjork-Sheily) for mitral stenosis (MS) associated with rheumatic heart disease 18 years ago, was admitted with worsening heart failure (HF) with progressive shortness of breath (PND and orthopnea) and leg edema for the past three weeks. Reportedly, she had thrombosis of the MV prosthesis requiring thrombectomy twice, 10 and 6 years ago, presumably due to non-compliance with warfarin therapy. Three years prior, she went into heart failure (HF) and was noted to be in atrial flutter (AFL). A malfunction of the MV prosthesis was suspected, but she declined MV replacement and refused catheter ablation of AFL. Since then, she has been in chronic HF (NYHA functional class III). At Emergency Department (ED), she was hypotensive and in severe respiratory distress with biventricular HF (BP 85/50mmHg, RR 26/min, PR 120/min, JVD with JVP 14 cm H20, RV and LV lifts with PMI displaced 2 cm to the left, no significant murmurs, S3 audible, diffuse crackles at ½ of posterior lung fields, and bilateral leg edema). On auscultation, opening and closing clicks of the MV prosthesis were markedly diminished. Subsequent echocardiography showed severe MS with MV area 0.7 cm2 (estimated diastolic gradient 26 mmHg), dilated LA, RA, and RV, PA systolic pressure (RVSP) 78mmHg, mild AR and severe TR, and adequate LV contractility. Notably, there was a thrombus or vegetation in the MV prosthesis. Pertinent laboratory data: Hb measured 11.0 g/dL WBC 8850 /uL, Plt 223K, BUN 32 mg/dL, Cr 1.16 mg/dL, PT 33.3/uL (INR 2.98), hCRP 1.83. Liver enzymes and electrolytes were within normal limits, and so were thyroid hormones (TSH 2.298 [N 0.40-4.0] uIU/mL, Free T4 1.43 [N 0.89-1.76] ug/dL). ABG showed pH 7.41 (N 7.35-7.45), pCO2 25 (N 32-45) mmHg, pO2 117 (N 75-100) mmHg (FIO2 100%), HCO3 15.8 (N 20-26) mmol/L, BE -7 (N -2.0 – +2.0) mmol/L, O2 Sat 99%. Serial blood cultures were negative. After intensive treatment, her HF gradually improved. Given adequate anticoagulation therapy and under the impression of malfunction of an old aged MV prosthesis with thrombus obstruction, urgent referral to a Heart Valve Team was in order.
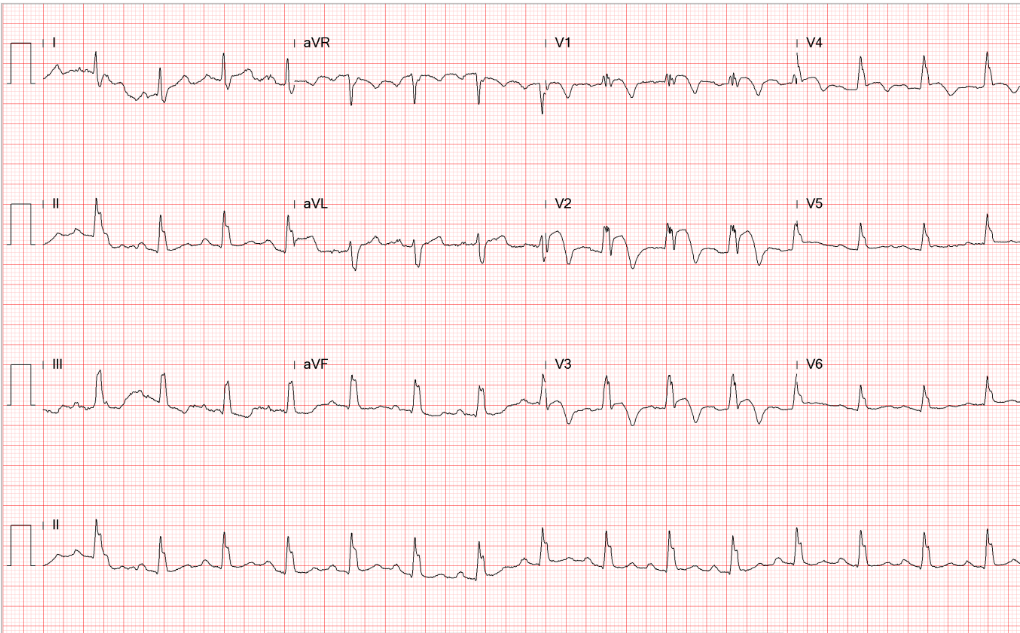
Diffuse low voltage
Atrial tachycardia or slow AFL (atypical form) with 2:1 A-V block (ventricular rate 123/min)
ST depression of >1mm in V4-V6 and ST elevation of <1mm in aVR c/w LV strain/subendocardial ischemia

Diffuse low voltage
LVH
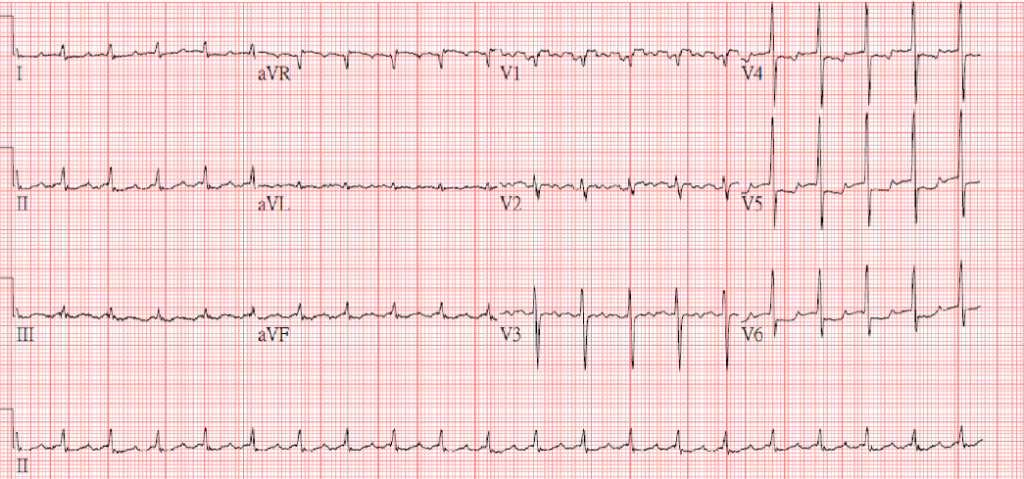
Atrial tachycardia or slow AFL (atypical form) with 4:1 A-V block (ventricular rate 65/min)
T inversion in V1-V3, ST depression of <1mm in V4-V6 suggestive of subendocardial ischemia or strain
The ventricular rate and the subendocardial ischemia or strain pattern have markedly improved compared to the previous tracing.
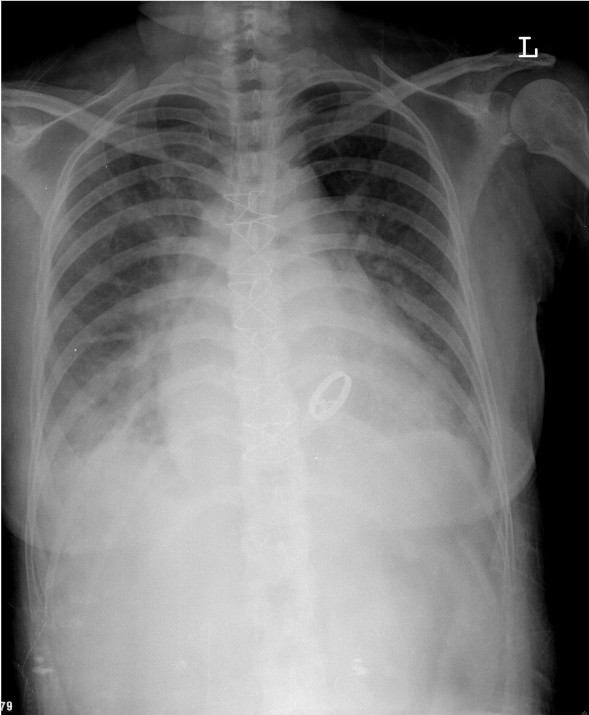
LV enlargement
RA and LA enlargement (note widened carina angle, bulge below left PA, double atrial density)
Prominent PA c/w PH
Pulmonary congestion with blunted bilateral costophrenic angles (pleural effusion)
A visible MV prosthesis (Bojork-Shiely)
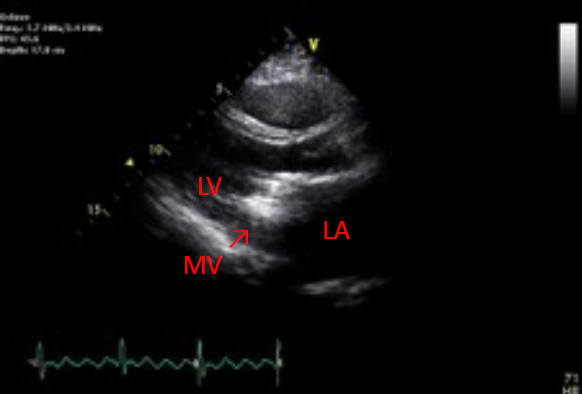
Dilated LA, RA, and RV
Adequate LV contractility with paradoxical septal motion
Severe MS with MV area measured 0.7 cm2
(PHT) with an estimated diastolic gradient of 26mmHg
Severe PAH (RSVP: 78mmHg)
Trace PR, mild to moderate AR, and severe TR
Rule out thrombus (arrow) or vegetation in MV prosthesis
The atrial tachycardia (or slow atypical AFL) with a 2-to-1 AV block is among the tachyarrhythmias (e.g., AFL, SVT) readily amenable to low-energy (<50 joules) electrical cardioversion. However, due to the intracardiac thrombus, converting the arrhythmia to a sinus rhythm is not advisable for fear of embolic stroke. Instead, controlling the ventricular rate as part of treating HF is the correct stratagem. Secondly, the ECG pattern of LV subendocardial ischemia is related to hypotension (BP 85/50mmHg) upon arrival at ED, as it markedly improved after treatment of HF with normalization of vital signs. However, the additional presence of significant atherosclerotic coronary heart disease remains likely.
Based on echocardiographic findings, ruling out infective endocarditis is of priority. After serial negative blood cultures and the absence of signs and symptoms of infection, intracardiac thrombus becomes the presumed diagnosis. Echocardiography can be used to monitor the function of prosthetic valves. It is recommended that transthoracic or transesophageal echocardiography with Doppler imaging be obtained six weeks to 3 months following implantation so that baseline information (e.g., structure, valve area, gradient, and transvalvular velocity, etc.) would be available for future comparison. In the present case, the prosthetic mitral valve is over 18 years old, and the MV thrombus seems to have obstructed the prosthetic valve (MV area 0.7 cm2 with estimated diastolic gradient 26 mmHg); accordingly, the Heart Valve Team was consulted for the thrombus removal and valvular replacement. Otherwise, in general, in the case of a non-obstructing thrombus, systemic anticoagulation with heparin can be first tried to dissolve the thrombus.
Malfunction of the prosthetic valve is among the myriad causes of worsening HF in patients with compromised cardiac function. These also include dietary indiscretion, medical non-compliance, volume overload (e.g., IV fluids or blood transfusion), deterioration of renal function, active myocardial ischemia, anemia, infection (e.g., pneumonia, endocarditis), cardiac arrhythmias (e.g., AF, VT, severe bradycardia), endocrine disorders (e.g., hyperthyroidism), negative inotropic drugs (e.g., antiarrhythmic drugs), etc. Searching out the cause of worsening HF is essential in preventing its recurrence.
Keywords:
echocardiography, infective endocarditis, intracardiac thrombus, rheumatic heart disease
UpToDate:
Management of mechanical prosthetic valve thrombosis and obstruction

This 43-year-old woman was rushed to ER because of the sudden onset of substernal chest tightness accompanied by cold sweats, which lasted more than 30

This 86-year-old woman with a long-standing history of hypertension, COPD, anemia, and hypertrophic obstructive cardiomyopathy (HOCM) was brought to the emergency department complaining of SOB

This 75-year-old man came to the Emergency Department (ED) complaining of abdominal pain for one day. He claimed he had constipation for several months, for
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.