
Case 24
An 88-year-old woman complained of a loss of appetite, generalized weakness for two weeks, and increasing SOB for five days. She had chronic heart failure
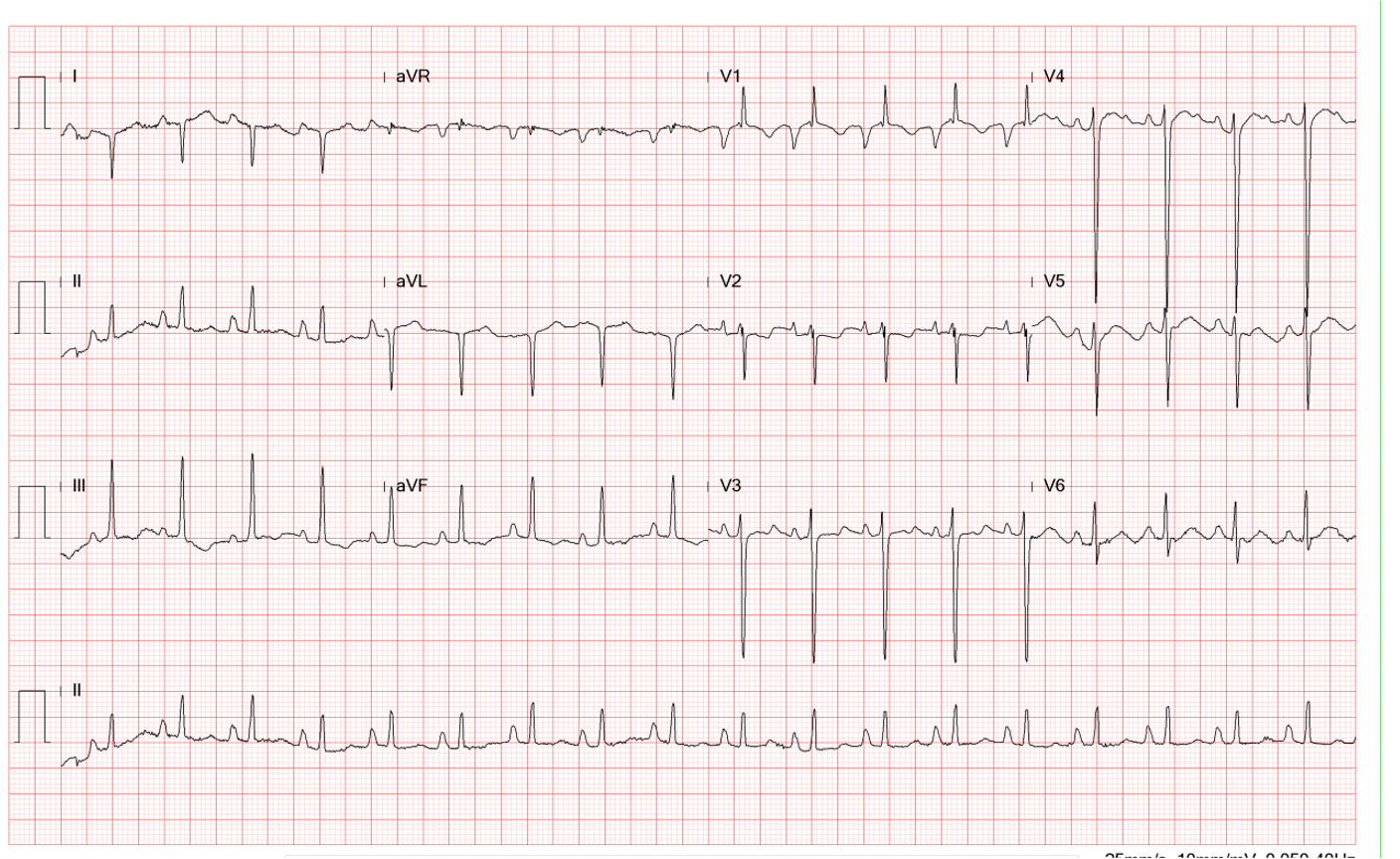
This 86-year-old woman with a long-standing history of hypertension, COPD, anemia, and hypertrophic obstructive cardiomyopathy (HOCM) was brought to the emergency department complaining of SOB associated with general malaise and productive cough with yellowish sputum for one week. She denied fever, chills, or chest pain but did have PND, orthopnea, and bilateral lower leg edema. When seen, she was in acute respiratory distress with BT/PR/RR: 36.9 ºC/99/min/26/min. BP measured 123/73 mmHg. Physical findings showed biventricular heart failure: JVD (10 cm H20) and PMI markedly displaced to the left anterior axillary line with a Gr4/6 systolic murmur at the apical area, crackles at both anterior and posterior lung fields and 3+ leg edema in both legs. ECG showed sinus tachycardia at 102/min, LA enlargement, first-degree degree AV block (PR 276 msec), complete LBBB, and likely LVH. Chest X-ray revealed cardiomegaly, mitral ring calcification, pulmonary hypertension (PH), and pulmonary congestion with infiltrates suggestive of pneumonia. SpO2 measured 84.5 (N 95-100) % with ABG showing pH 7.467 pO2 45.3 (N 75-100) mmHg, pCO2 32.8 (N 32-45) mmHg, HCO3 23., BE 0.2. Other pertinent laboratory data included Hb 10.2 g/dl, WBC 12,658 with a left shift (12% band 74%seg), PLt 325 x103, BUN 40.5 mg/dl, Cr 1.5 mg/dl troponin-I 0.27 ng/ml, CKMB 3.7 ng/ml, Na 146 mEq/L, K 3.8 mEq/L, CRP >25 mg/dl, SCPT 18 IU/L, NT-ProBNP >2500 (N <500 for >75 years old) pg/ml. Urinalysis showed sp. g. 1.103, yellowish clear, RBC 2-5/phf, WBC 2-5/phf, blood 2+, bacteria 3+.
Echocardiography confirmed HOCM with a peak LV outflow gradient of 65 mmHg, preserved LV contractility (EF 62%), sclerotic aortic valve, mitral ring calcification, and PH with mild TR. After collecting samples for culture, the care team empirically started antibiotic therapy with levofloxacin, mucolytic, diltiazem, and diuretics. Subsequent sputum grew out of mixed flora. Despite her age and severe comorbidities, she declined to sign a DNR (do not resuscitate). Throughout the course, she remained afebrile, and biventricular HF gradually improved (Chest- X-ray 2). The care team managed to discharge her in 8 days.

Sinus tachycardia at 102/min
1st degree AV block (PR 276 msec)
LA enlargement
Clockwise rotation of the heart likely due to COPD
Complete LBBB with QRSd of 150 msec
Left axis deviation (QRS axis -54º), with a T wave axis of +111º and an R wave amplitude of 20
mV suggestive of LVH
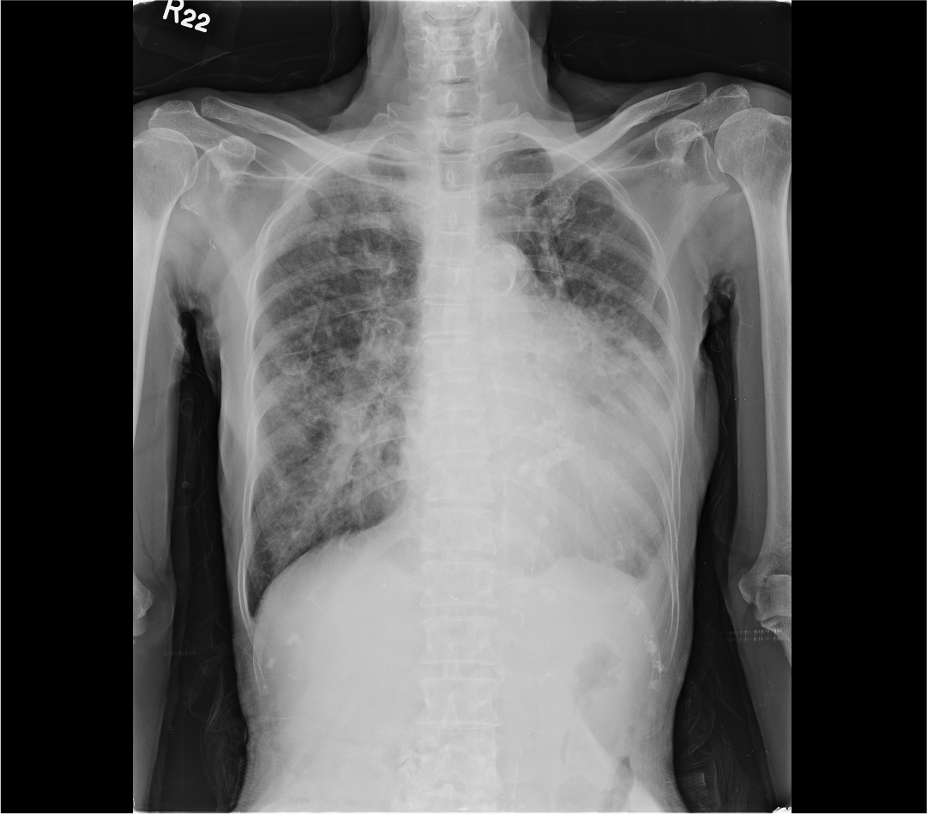
Emphysematous lungs (COPD)
Cardiomegaly with LA enlargement
Aortic knob calcification
Prominent PA indicative of PH
Pulmonary congestion suggesting HF
Pulmonary infiltrates likely due to pneumonia
Mitral ring calcification.
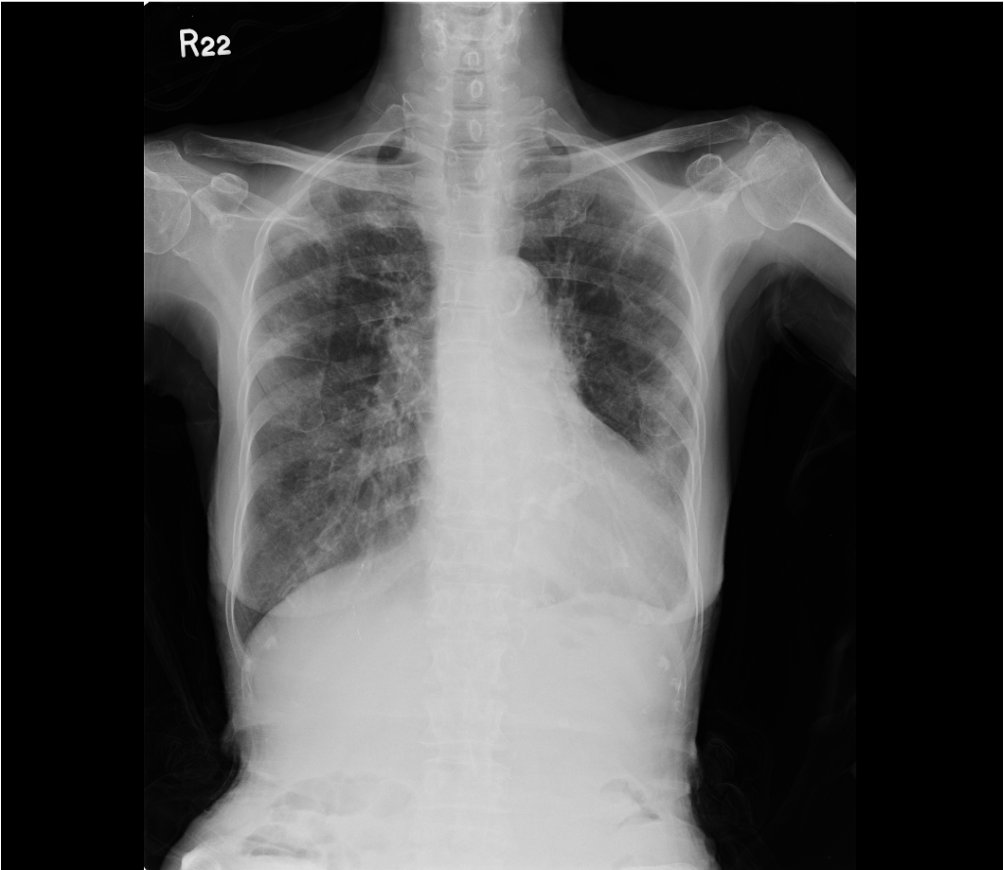
A marked improvement after therapy
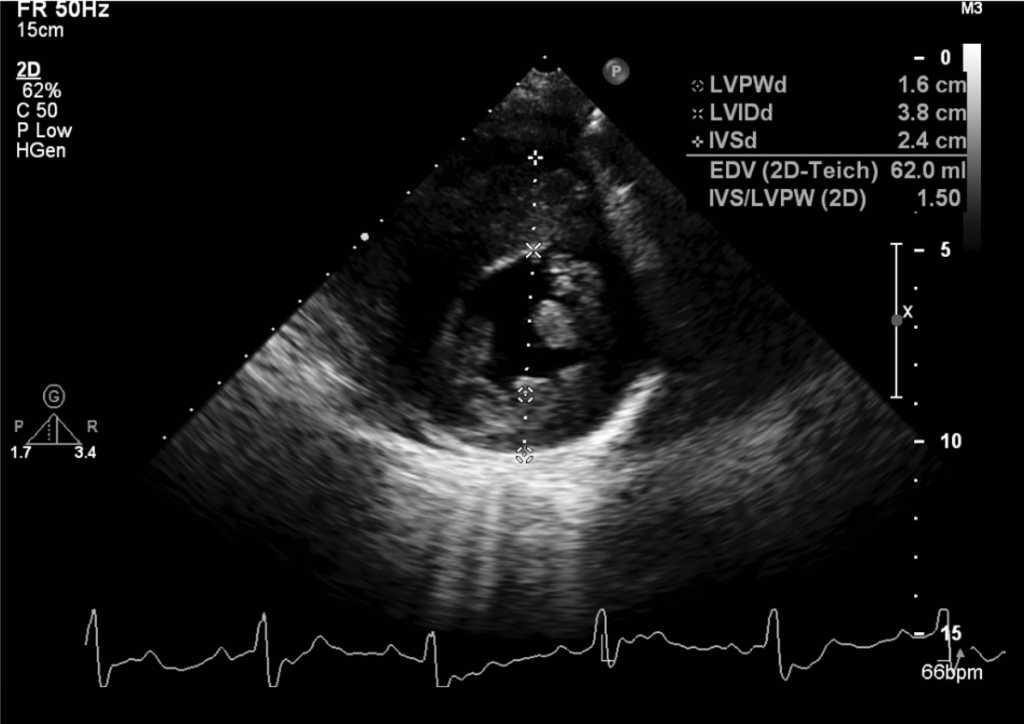
LVH with peak LV outflow gradient of 65 mmHg
Preserved LV contractility (EF 62%)
Aortic valve sclerosis with moderate AR
Mitral annulus calcification with moderate MR
PH with mild (TRPG 30 mmHg
Mild PR
The ECG shows sinus tachycardia with a complete LBBB pattern, which can obscure the typical diagnostic criteria for LVH due to its altered depolarization and repolarization processes. However, specific features can raise suspicion of LVH being present. For example, increased amplitude of the R-wave in the lead aVL, QRS duration >150ms, an R peak time >60ms in lead V5, and an aVL lead with QRS-T angle >30° are predicative of LVH in complete LBBB. However, these ECG findings have varying sensitivity and specificity and, thus, are not the gold standard for diagnosing LVH in LBBB. Echocardiography remains the most commonly used modality, as it allows for accurate measurement of LV mass and identification of LVH even in the presence of LBBB. Cardiac MRI can also be employed to measure LV mass, wall thickness, and myocardial fibrosis precisely, a common feature of LVH.
As above, in the present case, the findings of QRSd of 150 msec, the R wave amplitude of 20 mV, the left axis deviation (QRS axis -54°), and the T wave axis of +111° favor the presence of LVH in complete LBBB as confirmed by echocardiography. COPD further complicates the ECG findings, as it results in the slow progression of R waves in the precordial leads (clock rotation of the heart), reducing the R wave amplitude in leads V5 and V6. The finding of the first-degree A-V block is intriguing. Although it may represent an adverse effect of calcium channel blocker therapy, it may indicate myocardiopathy involvement of the A-V node, His bundle, and the right bundle branch alongside complete LBBB. In the latter situation, the risk of developing a complete heart block should be taken into consideration while managing the overall context of HOCM, COPD, and anemia in this patient.
Hypertrophic cardiomyopathy (HCM), whether obstructive or non-obstructive, frequently presents with specific ECG changes. However, it is essential to note that ECG findings are not definitive for diagnosing HCM but warrant further evaluation with imaging studies like echocardiography or cardiac MRI. Typical ECG findings in HCM are 1. Voltage criteria for LVH are recognizable, including the increased amplitude of R or S waves across the precordial leads, deep S wave in lead V1 or V2, or tall R wave in leads V5 or V6. 2. Abnormal Q waves in the inferior and lateral leads are unrelated to coronary artery disease and can help differentiate from LVH due to other causes. 3. T-wave Inversion is often seen in the left precordial leads, particularly leads V5 and V6. 4. Atrial fibrillation may occur in patients with HCM due to the enlarged LA; ventricular arrhythmias like PVCs or non-sustained ventricular-tachycardia may be present. 5. Bi-atrial enlargement can be suggested by tall, peaked P waves in the lead II due to RA enlargement and widened, notched P waves in the lead II or a deep negative P wave deflection in the lead V1-2 due to LA enlargement. 6. QRS complex abnormalities, e.g., left or right axis deviation and nonspecific intraventricular conduction delay, are identifiable. Clinicians should know that ECG does not directly differentiate between obstructive and non-obstructive HCM. This distinction is typically made on echocardiography with Doppler or cardiac catheterization by evaluating the presence or absence of left ventricular outflow tract (LVOT) obstruction. Clinicians must always correlate ECG findings with the clinical context, and when HCM is suspected based on ECG (or symptoms), an echocardiography must be ordered to confirm the diagnosis.
A mutation of the sarcomere genetically causes HCM. HOCM occurs when there is an asymmetric thickening of the heart, most commonly of the interventricular septum, leading to the narrowing of the LVOT and subsequent obstruction. In symptomatic patients, HOCM can also be revealed by physical examination findings, which commonly include a harsh systolic murmur at the left lower sternal edge that increases on Valsalva maneuver and diminishes on squatting. Echocardiography is the most widely used diagnostic tool. It shows LVH, particularly of the interventricular septum. The hallmark is the mitral valve’s systolic anterior motion (SAM) leading to left ventricular outflow tract (LVOT) obstruction. ECG can show LVH with ST-T wave changes and sometimes arrhythmic patterns, which can aid in the suspicion of underlying HOCM. Cardiac Catheterization is used mainly when findings from non-invasive modalities are insufficient to make a diagnosis. Cardiac MRI can be helpful to characterize the pattern and extent of hypertrophy and evaluate for myocardial fibrosis.
The first line of treatment for HOCM commonly involves beta-blockers, which help reduce heart rate, increase filling time, and lower the degree of LVOT obstruction. If beta-blockers are unsuccessful or contraindicated due to COPD, as in the present case, nondihydropyridine calcium channel blockers (e.g., verapamil and diltiazem) are often tried. If medical therapy fails, Surgical myectomy or alcohol septal ablation can be considered. Implantable Cardioverter-Defibrillators (ICDs) are considered for primary prevention in patients with risk factors (positive family history, unexplained syncope, etc.) for sudden cardiac death. HOCM patients should avoid strenuous physical activity or competitive sports and stay well-hydrated to maintain adequate volume. As HCM is a genetically inherited disease, genetic counseling should be considered for the patient and first-degree relatives. Anticoagulation and rhythm control strategies are essential in atrial fibrillation cases, as they frequently complicate HOCM.
Notably, the FDA has recently approved a new drug called mavacamten (Camzyos) to treat adults with symptomatic NYHA class II-III HOCM. By inhibiting the activity of cardiac myosin, mavacamten reduces excessive cardiac contractility, thereby mitigating the extent of LVOT obstruction in HOCM. However, patients who have severe concurrent comorbidities such as pneumonia, atrial fibrillation, or VT are at greater risk of developing impaired heart muscle contraction, leading to HF with mavacamten. Because of the risk of HF, patients treated with mavacamten must be monitored with echocardiograms; patients should also avoid specific medicines that interfere with its pharmacokinetics. Currently, mavacamten is only available through a restricted program called the Camzyos Risk Evaluation and Mitigation Strategy (REMS).
Keywords:
chronic obstructive lung disease (COPD), complete left bundle
branch block (LBBB), hypertrophic cardiomyopathy (HCM),
mavacamten
UpToDate:
Hypertrophic cardiomyopathy: Clinical manifestations, diagnosis and
evaluation
Hypertrophic cardiomyopathy: Management of patients with outflow
Tract obstruction.

An 88-year-old woman complained of a loss of appetite, generalized weakness for two weeks, and increasing SOB for five days. She had chronic heart failure
This 69-year-old man had been in good health until two days before admission when he returned from a beach. He experienced general weakness with chest
A 56-year-old man came to the Emergency Department because of worsening dyspnea with a productive cough for the past two weeks. Notably, he smoked one
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung all right Reserved. Designed by 青澄設計 Greencle Design