Case 10
A 43-year-old woman, s/p mitral valve (MV) replacement (Bjork-Sheily) for mitral stenosis (MS) associated with rheumatic heart disease 18 years ago, was admitted with worsening
This 35-year-old male was transferred from a local hospital for further evaluation after a biopsy of a hard, 4 cm in diameter lymph node in the left supraclavicular fossa (Virchow’s node)* suggestive of metastatic malignancy. His chief complaint was progressive SOB with a productive cough for the past week despite having received rifampicin and ethambutol therapy for recently diagnosed pulmonary tuberculosis (TB) for about one month. He claimed to have lost 8 kg in the past six months. He appeared apprehensive in acute respiratory distress, using accessory muscles for breathing, but remained mentally alert. BT/PR/RR measured 36.9 ℃/115bpm/30cpm, and BP was 104/74 mmHg. Jugular venous distension (JVD) was estimated to be 10 cm H2O. A scar s/p removal of the lymph node was noted. The PMI was not visible. Heart sounds were distant, breathing sounds were diminished, and there were no crackles or wheezing in either lung field. The liver was four-fingerbreadth palpable below the right costal margin. The rest of the physical findings were unremarkable. ECG showed sinus tachycardia at 110/min, diffuse low voltage, and electrical alternans. Chest X-ray revealed cardiomegaly and widespread interstitial process (reticular pattern of infiltrates with nodules), r/o lung cancer, and pneumonia. Laboratory data included Hb 15 g/dL, Hct 44.7%, and WBC 11,450, BUN 11.6 mg/dL Cr 0.9 mg/dL, SGOT 78 (N 0-40) U/L, SGPT 42 (N 0-40) U/L, ALP 72 (N 37-95) U/L, uric acid 8.5 (2.7-8.5) mg/dl, Na 138 mEq/L, K 4.6 mEq/L, CRP 2.32 (N <0.5) mg/dl. Being anxious to rule out lung cancer, the care team first did a chest CT, which revealed not only a lung tumor at the right upper lung but also a significant amount of pericardial effusion as suggested by ECG findings (diffuse low voltage with electrical alternans). The care team then used a manual sphygmomanometer to measure the paradoxical pulse,**estimated to be at 20 mmHg. A real-time echocardiography subsequently confirmed cardiac tamponade, as it demonstrated RV diastolic collapse surrounded by a substantial amount of pericardial effusion. Accordingly, the care team performed a pericardiocentesis on an emergency basis and removed about 1000 cc of pericardial fluid. It was an exudate, turbid containing protein 4.6 g/dL, LDH 310 u/L, acid-fast stain (-), and few atypical cells, reported as probably metastatic carcinoma in nature. Following pericardiocentesis, the patient felt more comfortable as it relieved much of the breathing difficulty (Chest X-ray 2). Later, the results of the lymph node biopsy showed adenocarcinoma of the lung. After consultation with the chest and oncology specialists, the care team started erlotinib (Tarceva). This tyrosine kinase inhibitor acts on the epidermal growth factor receptor (EGFR) as the initial therapeutic agent for lung cancer.
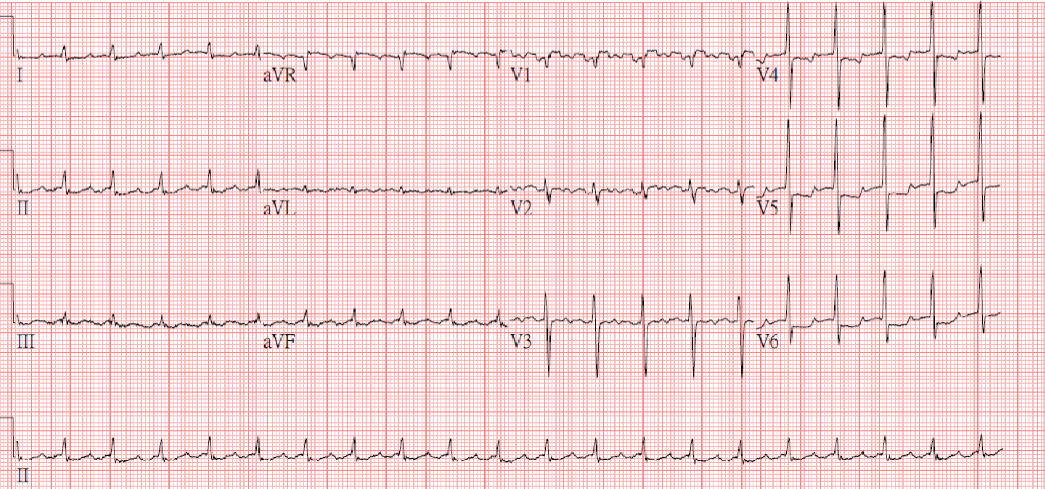
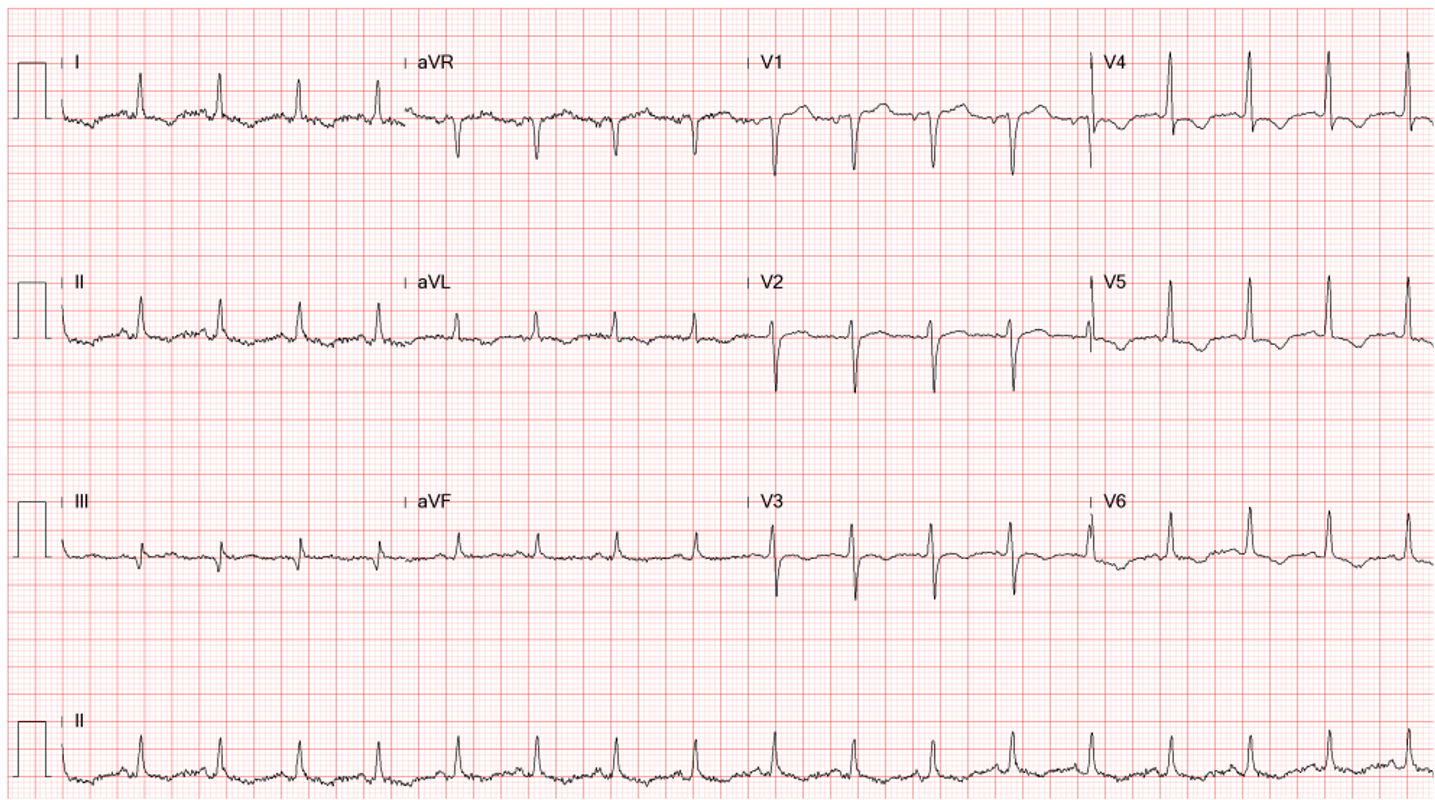
Diffuse low voltage
Sinus tachycardia at 110/min
Diffuse nonspecific ST-T changes
Electrical alternans, especially in leads V2-V4

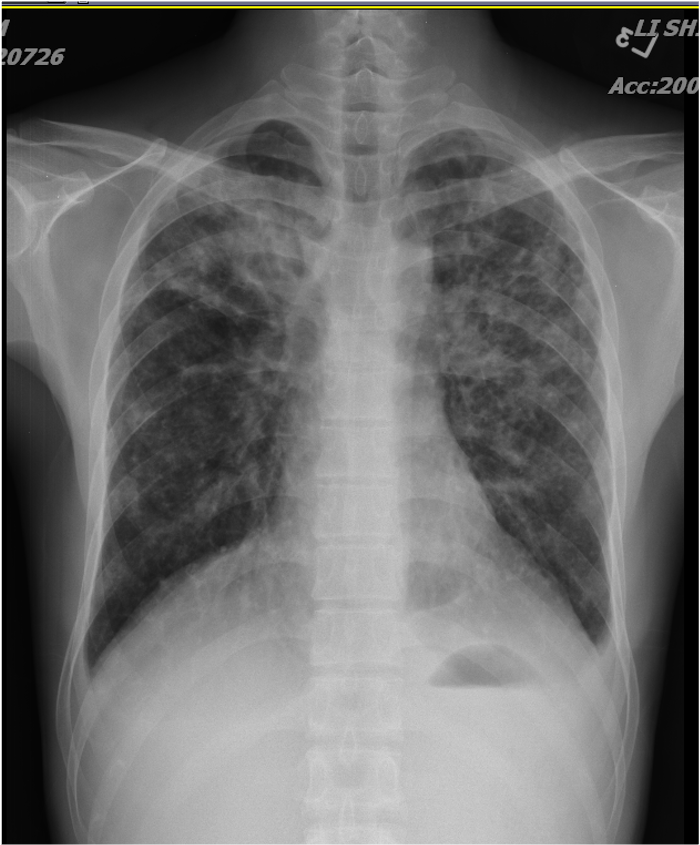
Increased cardiac silhouette suggestive of cardiomegaly
A reticular pattern of infiltrates (in both lungs) and nodular lesions (in the right upper lung field),
suggesting an interstitial process, r/o lung cancer, and secondary infection like pneumonia.
The previous “cardiac silhouette suggestive of cardiomegaly” is no longer seen.
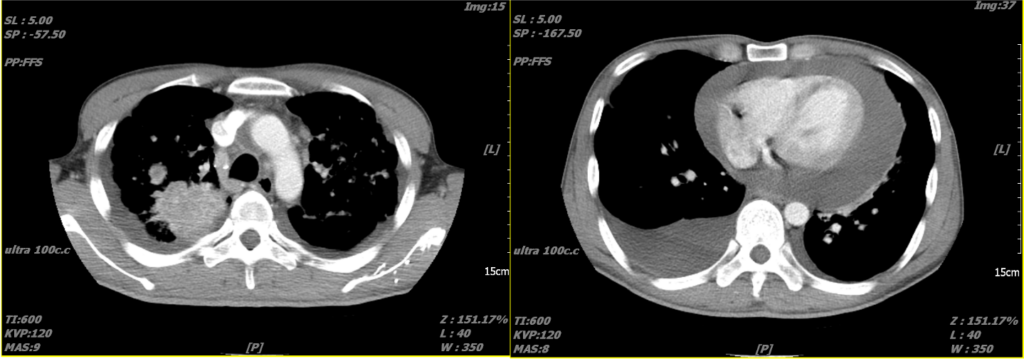
An immense amount of pericardial effusion
A lung tumor in the upper right lung
Pleural effusion in the right posterior lung field
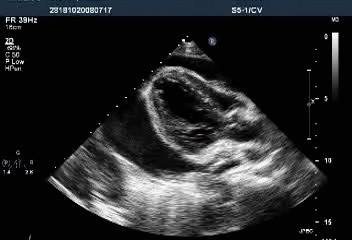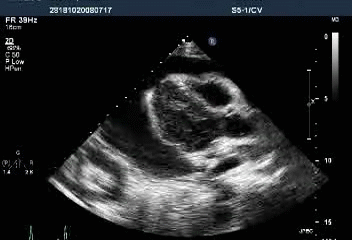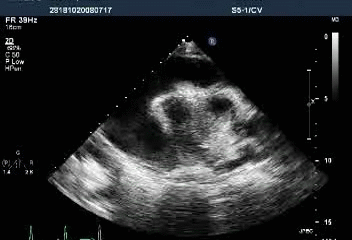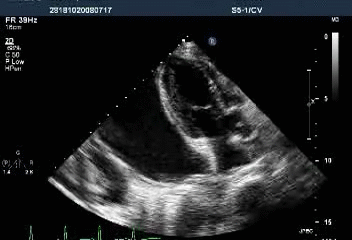
Massive pericardial effusion with a swinging heart
Cardiac tamponade with diastolic RV collapse
Exaggerated respiratory pattern of mitral and tricuspid inflow
Preserved LV systolic function (LVEF 89.58%)
The ECG findings of diffuse voltage, electrical alternans, and ST-T changes resonate with substantial pericardial effusion. However, echocardiography is the gold standard to confirm the diagnosis of pericardial effusion as these ECG changes are relatively nonspecific. Specifically, besides pericardial effusion, clinical conditions that can produce diffuse low voltage include obesity, chronic obstructive pulmonary disease (COPD) or pulmonary emphysema, restrictive or dilated cardiomyopathy, infiltrative myocardial diseases (e.g., amyloidosis and sarcoidosis), myocarditis or myocardial infarction, endocrine disorders (e.g., hypothyroidism, and adrenal insufficiency), chest wall edema, and advanced age (due to fibrosis and scarring of the myocardium), etc. On the other hand, electrical alternans may be seen in conditions other than pericardial effusion. These include severe LV dysfunction, cardiac sarcoidosis, severe pulmonary hypertension, tachyarrhythmias (i.e., VT and SVT), severe myocardial ischemia or infarction, digoxin toxicity, septic shock (with cardiac instability), among others.
Various conditions can clinically manifest with pericardial effusion, in severe cases, leading to cardiac tamponade: 1. Pericarditis (e.g., viral, bacterial, or autoimmune pericarditis), 2. Advanced malignancies, particularly lung cancer and breast cancer, as well as lymphomas and leukemias, 3. Blunt chest trauma. 4. Post-cardiac surgery, 5. Aortic dissection with rupture into the pericardial space causing a hemopericardium and tamponade. 6. Myocardial rupture myocardial infarction with myocardial rupture and consequent tamponade. 7. Tuberculosis 8. Uremic pericarditis (chronic renal failure) 9. Drug-induced lupus syndrome (e.g., hydralazine, isoniazid, and procainamide), 10. Autoimmune conditions: SLE, rheumatoid arthritis, and scleroderma, 11, cardiac procedure-related injuries such as cardiac catheterization and cardiac device implantation. 12. Lastly, in many instances, pericardial effusion is idiopathic, as no specific cause can be found.
As for the etiological work-up, laboratory tests such as CBC, cardiac enzymes, sedimentation rate, CRP, pericardial fluid analysis and culture, imaging with CT scan, and lymph node biopsy are necessary, as shown in the present case. In essence, clinicians must interpret the ECG findings within the clinical context described in the “five-finger approach” method.***
Cardiac tamponade refers to heart compression due to fluid accumulation in the pericardium, which impedes diastolic filling and decreases cardiac output. The speed and degree of fluid collection often determine the severity of symptoms, categorizing the condition as acute, subacute, or chronic. Acute cardiac tamponade is characterized by an abrupt accumulation of fluid in the pericardial space, often secondary to trauma, aortic dissection, or cardiac rupture (e.g., a complication of acute MI). Due to sudden stretching of the parietal pericardium, where parasympathetic nerve endings reside, acute cardiac tamponade can induce sudden onset of sinus bradycardia, A-V junctional rhythm, and even complete heart block. In contrast, sinus tachycardia is usually seen in chronic and subacute cardiac tamponade, as in the present case.
Clinically, Beck’s triad suggestive of cardiac tamponade includes hypotension, JVD, and muffled heart sounds. However, in light of ECG’s showing diffuse low voltage and electrical alternans, the care team should have checked the presence of pulsus paradoxus or directly ordered echocardiography to rule out the existence of pericardial effusion and cardiac tamponade even without clinical hypotension (BP 104/74 mmHg). Typical echocardiographic findings are a swinging heart within the fluid-filled pericardium and diastolic collapse of RA and RV, as illustrated in the present case. After the stabilization of the patient’s condition, a pericardiocentesis is deployed to remove the pericardial fluid. In more severe cases or where malignancy or recurrent effusion is suspected, a pericardial window (removal of a part of the pericardium) may be performed to allow fluid to drain into the chest cavity. All therapeutic interventions should be coupled with treatment of the underlying cause of the tamponade, where possible, to prevent recurrence. Cardiac tamponade is a life-threatening emergency requiring early diagnosis and management. Prompt recognition and management of cardiac tamponade is of crucial importance due to its potential for rapid progression to life-threatening hemodynamic instability.
Adenocarcinoma is the most common type of lung cancer in nonsmokers and women. It originates from the cells that line the alveoli and bronchial passages of the lung, which have undergone malignant transformation, primarily seen in the peripheral areas of the lungs. It can vary in appearance from well-differentiated forms resembling normal lung architecture to poorly differentiated forms showing solid growth patterns: lepidic, acinar, papillary, micropapillary, and solid adenocarcinoma. Recent advances in treatment have significantly improved the survival rate. The treatment approach depends on several factors, such as the disease stage, the tumor’s molecular characteristics, the patient’s performance status, and overall health condition. 1. Surgical resection remains the first line of treatment for early-stage adenocarcinoma; lobectomy is the most common procedure, but segmentectomy or wedge resection can be performed for patients with limited pulmonary function. 2. Radiation therapy is applied in patients who are medically unfit for surgery, those who refuse surgical options, or in locally advanced cases; stereotactic body radiation therapy (SBRT) is often used in early-stage non-small cell lung cancer (NSCLC) in non-surgical candidates. 3. Chemotherapy (cytotoxic chemotherapy) is typically reserved for advanced-stage disease and adjuvant therapy in earlier stages after surgical resection. 4. Targeted therapies are for patients with specific genetic alterations. For example, tumors with EGFR mutations may be treated with tyrosine kinase inhibitors such as erlotinib, gefitinib, and afatinib. 5. Immunotherapy is for patients with advanced-stage disease with agents like pembrolizumab, nivolumab, or atezolizumab; these agents work by unleashing the body’s immune system to fight cancer. 6. Lastly, combination therapies may be deployed for patients with a specific situation. Multidisciplinary discussion involving medical oncologists, radiation oncologists, thoracic surgeons, pulmonologists, and thoracic radiologists is critical in determining the optimal treatment strategy for each patient.
*Virchow’s node, or Troisier’s sign, refers to the enlarged left supraclavicular lymph node, typically associated with metastatic carcinoma. It is named after Rudolf Virchow, a German pathologist who was the first to correlate an enlarged left supraclavicular node with malignancy. From a clinical perspective, detecting an enlarged Virchow’s node is significant as it usually indicates an underlying malignancy, often in the abdominal or thoracic region. The lymphatic drainage from these areas, including the stomach, lungs, pancreas, ovaries, kidneys, and prostate, among others, can culminate in the left supraclavicular node. As such, its enlargement may be an essential indication of a previously undetected, advanced metastatic disease. Assessment of a patient presenting with a Virchow’s node should include a thorough search for malignancy if other signs or symptoms suggest such a diagnosis. Given the association with advanced disease, the discovery of a Virchow’s node generally tends to indicate a poor prognosis. It is worth mentioning that clinical judgment will be crucial in these situations, as not every enlarged left supraclavicular node will be a Virchow’s node.
**Paradoxical pulse, or pulsus paradoxus, describes an exaggerated decrease in systolic blood pressure greater than ten mmHg during inspiration. Under normal physiological conditions, a slight drop in systolic blood pressure can occur during inspiration due to intrathoracic pressure changes. However, this normal variation can be amplified in certain pathological states, leading to pulsus paradoxus. Pulsus paradoxus is a significant clinical finding as it can provide essential clues toward certain medical conditions. It is often associated with conditions impairing the heart’s filling during diastole, particularly those affecting the right heart. Pulsus paradoxus is most commonly associated with cardiac tamponade, where fluid accumulation in the pericardial space impairs cardiac filling. Other conditions, such as severe asthma and chronic obstructive pulmonary disease (COPD), can also manifest pulsus paradoxus due to dynamic hyperinflation and increased negative intrathoracic pressure during inspiration. Pulsus paradoxus is measured using a manual sphygmomanometer by noting the pressure difference between the first audible sound (during expiration) and the point at which the sound is heard continuously (during both inspiration and expiration). Detection and quantification of pulsus paradoxus can help clinicians diagnose and assess the severity of these conditions.
***In the “five-finger approach,” clinicians consider history (present illness, past medical history, and current medications), ECG, chest x-ray, and laboratory data while evaluating a patient to make the diagnosis.
Keywords:
cardiac tamponade, pericardial effusion, pulsus paradoxus, lung cancer, Virchow’s node
UpToDate:
Overview of the initial evaluation, diagnosis, and staging of patients
with suspected lung cancer
Management of stage I and stage II non-small cell lung cancer
Personalized, genotype-directed therapy for advanced non-small cell
lung cancer

A 43-year-old woman, s/p mitral valve (MV) replacement (Bjork-Sheily) for mitral stenosis (MS) associated with rheumatic heart disease 18 years ago, was admitted with worsening

An 88-year-old woman complained of a loss of appetite, generalized weakness for two weeks, and increasing SOB for five days. She had chronic heart failure
This 50-year-old man came to the Emergency Department complaining of having substernal chest pain for more than 10 hours. The pain was sharp and not
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.