Case 28
This 92-year-old man complained of progressive dyspnea with PND, productive cough with whitish sputum, and bilateral leg edema for the past few weeks. He was
This 69-year-old man had been in good health until two days before admission when he returned from a beach. He experienced general weakness with chest tightness and shortness of breath (SOB) associated with watery diarrhea, nausea, and vomiting. There was no accompanying fever, chills, cough, sore throat, rhinorrhea, or dysuria. He denied any recent traveling. Past medical history was significant only for hyperlipidemia. On arrival at the Emergency Department, he looked apprehensive and was in respiratory distress. BP measured 84/51mmHg, and BT/PR/RR 36.6°C, 61/min, and 22/min, respectively. There was no gross cardiomegaly or murmur but audible S3 and bilateral crackles. The abdomen was soft and had no tenderness, rebounding pain, or shifting dullness; the bowel sound was hypoactive. ECG showed sinus rhythm at 66/min with diffuse low voltage; Q waves with ST-segment elevation in leads II, III, aVF, and V2-V6 suggested acute extensive myocardial infarction (MI). Pertinent laboratory data revealed ABG: pH 7.4, PaO2 59.6 mmHg, SaO2 91.4% (on O2 nasal cannula), Hb 14 gm%, WBC 4800, Plt 141 K, AST 322 (N 8-48) IU/L, ALT 249 (N 7-55) IU/L, CRP 2.66 (N <0.3) mg/dL, Na 113 (N 135-145) mEq/dL, K 4.6 (N 3.5-5.2) mEq/dL, BUN 26 mg/dL, Cr 1.2 mg/dL CKMB 50.9 (N 5-25) IU/L, troponin-I 10.8 (N 0-0.04) ng/ml, BNP 674 (N <100) pg/ml, d-Dimer 2490 (N 500) ng/ml, TSH 2.26 (N 0.5-5.0) mTU/L, and free T4 1.2 (N 0.7-1.9) ng/dL. Under the impression of acute coronary syndrome (ACS) with extensive MI, the care team decided on emergency coronary angiography. While transferring him to the cardiac catheterization laboratory, he suddenly had a cardiac arrest due to ventricular fibrillation (VF). He was resuscitated and inserted with an intra-aortic balloon pump (IABP). Surprisingly, except for the left circumflex artery being hypoplastic, there were no occlusive lesions in any coronary arteries. Notably, the LV was globally hypokinetic. Subsequent echocardiography exhibited all cardiac chambers of normal size and confirmed a globally hypokinetic LV with an EF of 32.6 %. Since the cardiac event had occurred after a few days of gastroenteritis (watery diarrhea with nausea and vomiting), the care team contained the diagnosis as a “clinically suspected” case of fulminant myocarditis (FM) of a viral etiology presenting as ACS. Accordingly, they immediately added oseltamivir (75 mg twice daily) to the medical regimen. Other relevant medications included atorvastatin, dobutamine, dopamine HCL, enoxaparin, spironolactone, and furosemide. Interestingly, during the course, serial ECGs showed changes in QRS voltage, degree of ST-segment elevation, and transient appearance of an ectopic atrial rhythm and the RBBB pattern. Nevertheless, the patient gradually improved, and even though the cardiac function remained compromised, he could return home in three weeks.
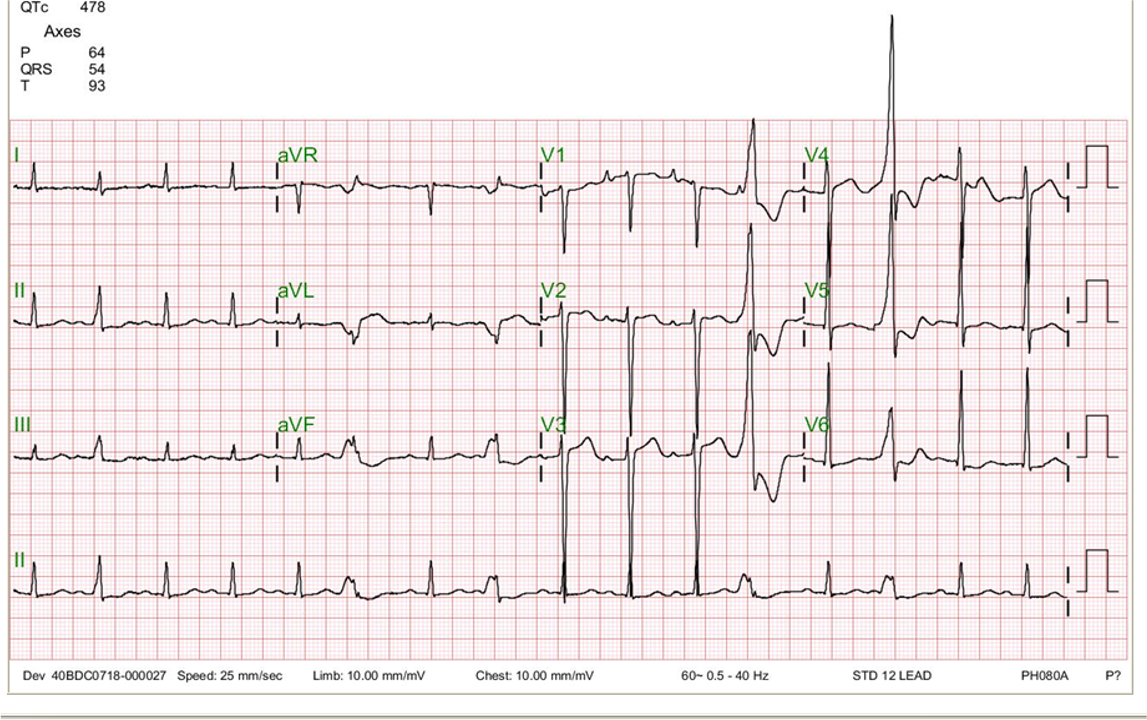
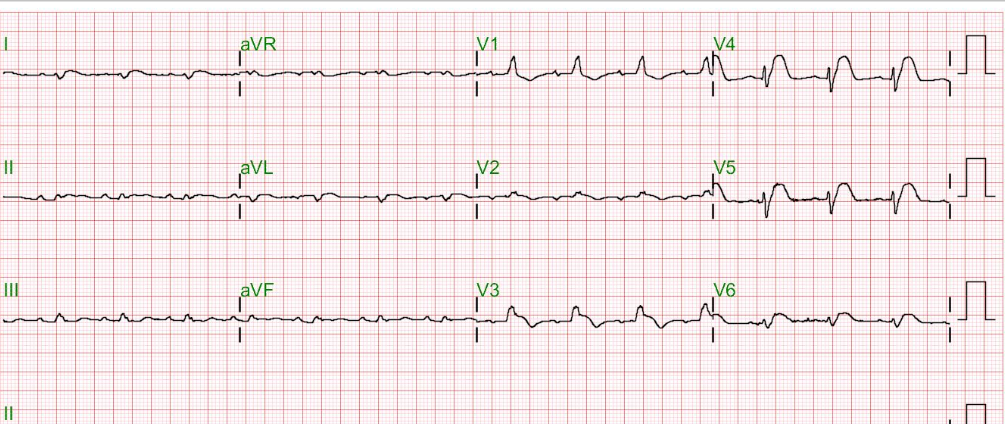
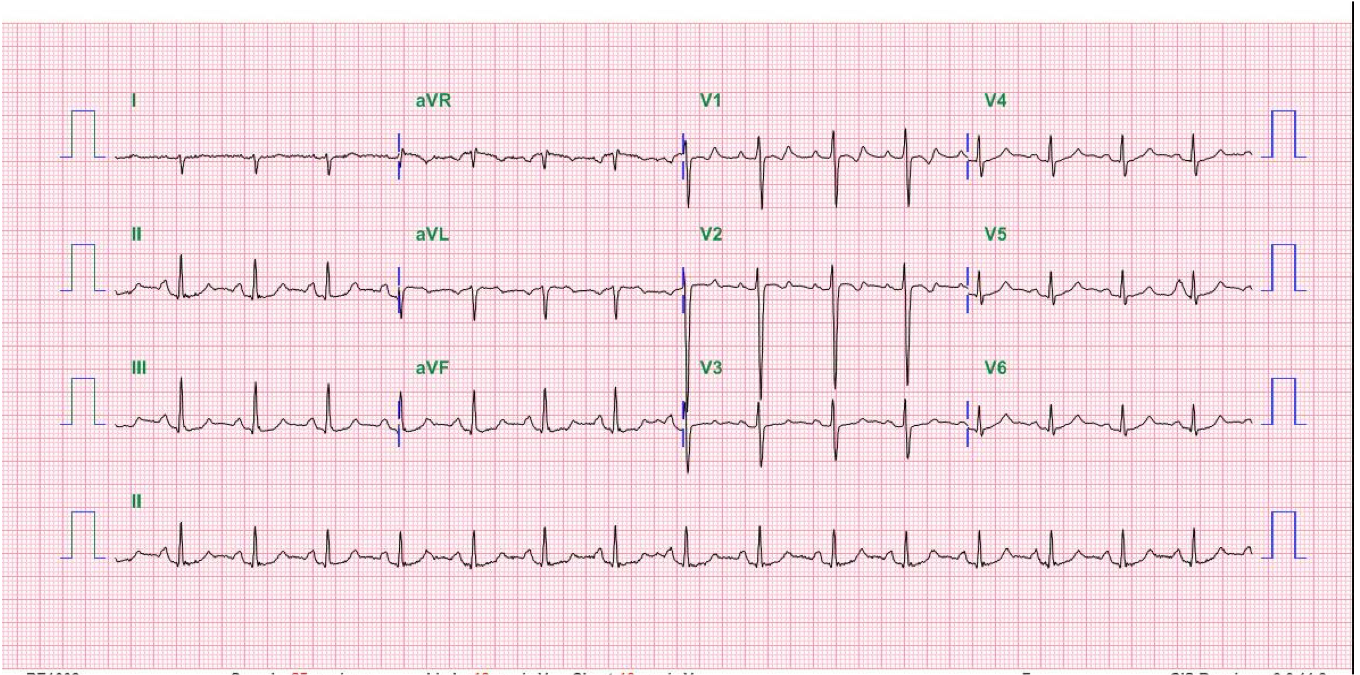
Sinus rhythm at 66/min
Diffuse low voltage
Left QRS axis deviation (-73°)
Q waves with ST-segment elevation in leads II, III, aVF, and V2-V6 suggestive of acute extensive MI
ST segment depression in aVR (a reciprocal change)
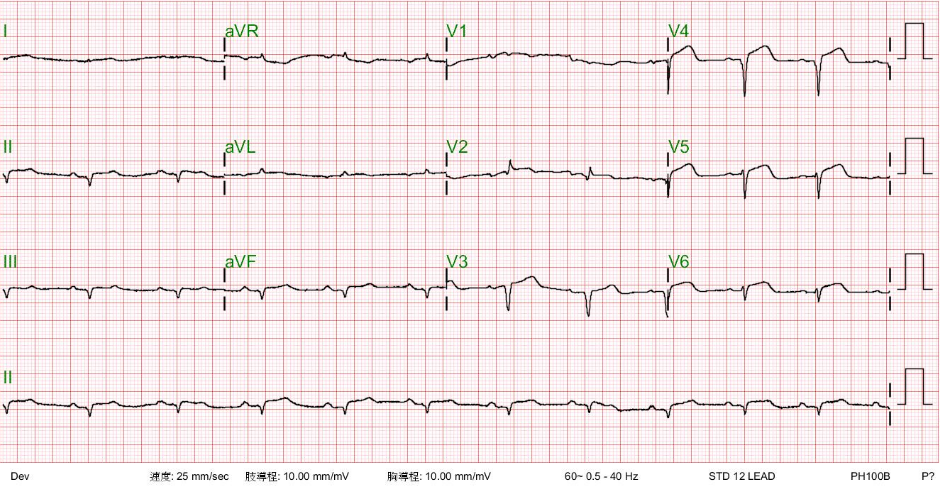
Sinus rhythm at 75/min
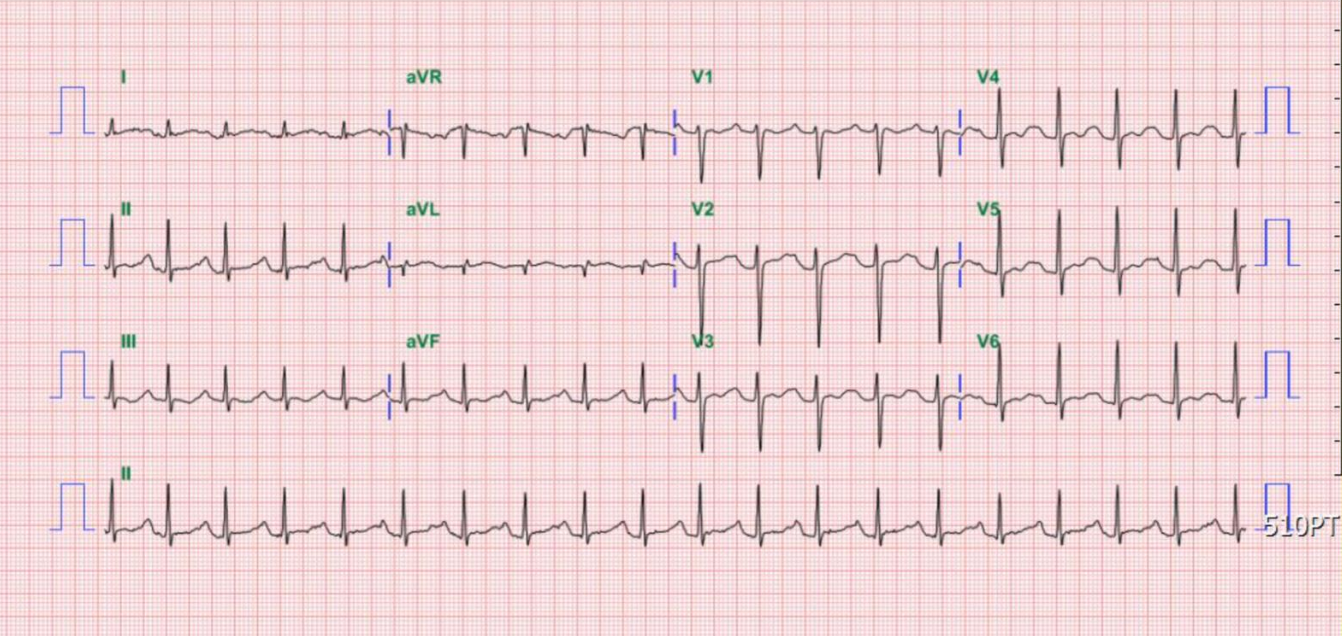
Compared to the one taken on admission, there is a further decline in the QRS voltage, especially in the precordial leads.
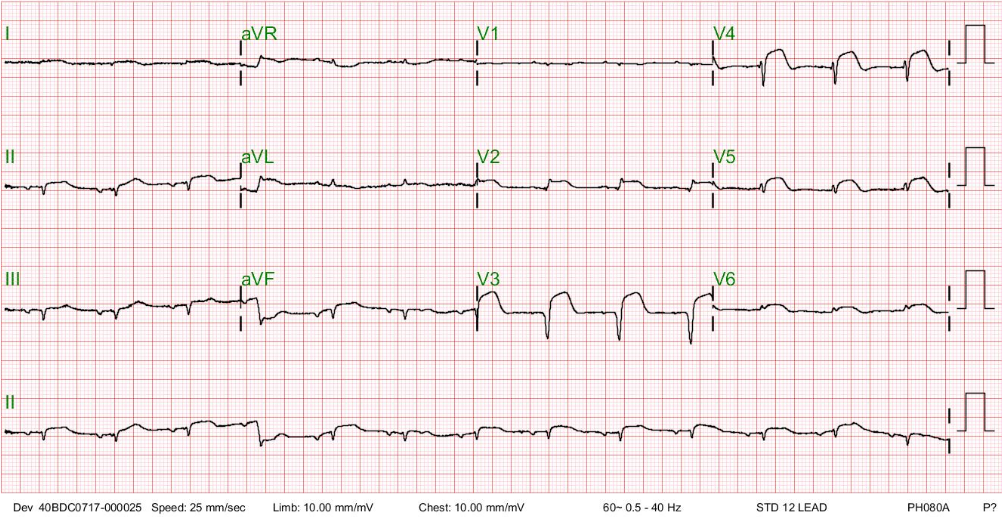
It is now an ectopic atrial rhythm at 80/min compared to the prior one. The QRS has regained the voltage; notably, there is more ST-segment elevation of the convex type.
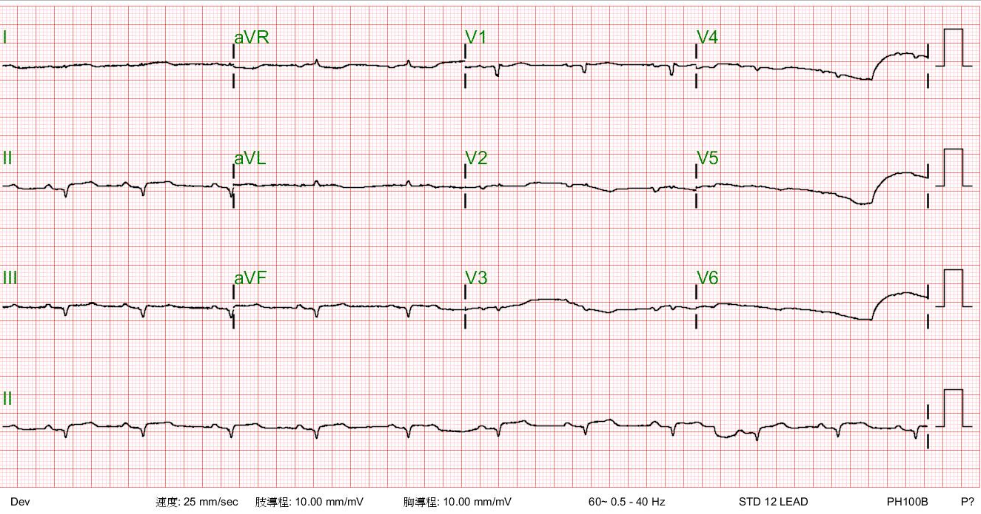
Compared to the previous tracings, sinus rhythm has resumed at 88/min
Diffuse low voltage has remained, and there is an incomplete RBBB pattern (QRSd 114 msec)
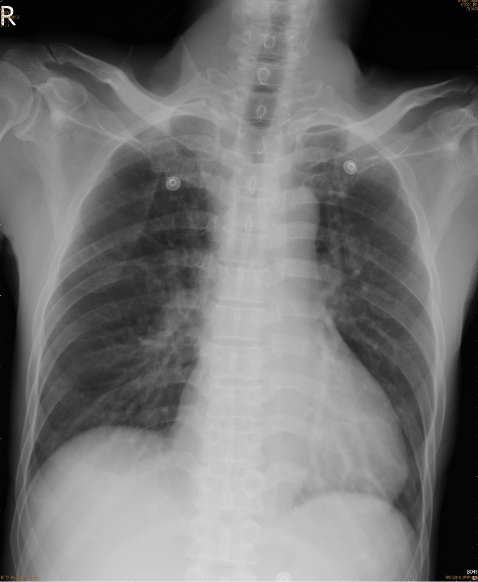
Prominence of bilateral hilar vascularities and lung markings.
Borderline cardiomegaly
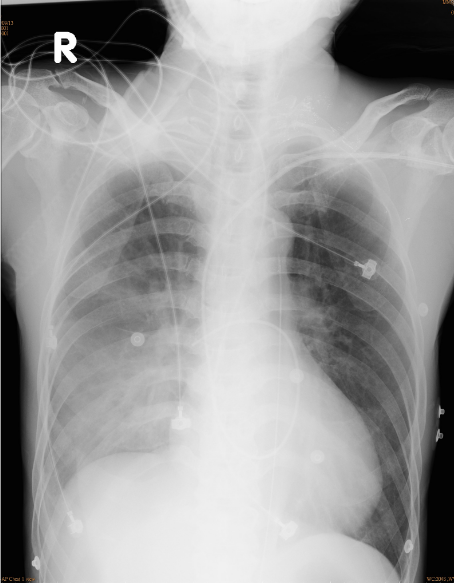
Marked infiltrates with effusion in the right lung after cardiac arrest
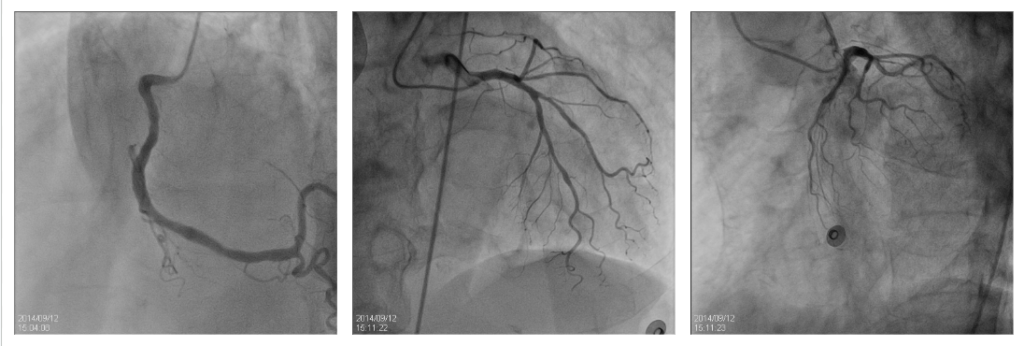
LCX hypoplasia.
No significant coronary lesions.
Normal sizes of cardiac chambers
Global LV hypokinesia with an EF of 32.6 %.
Mild MR, AR, TR.
The ECG findings of wide-spread Q waves (leads II, III, aVF, and V2-V4) with ST-segment elevation (leads II, III, aVF, and V2-V6), along with symptoms of chest tightness and SOB, had prompted the suspicion of ACS with extensive MI; According to the lead distribution, these findings indicate that all three major coronary arteries are simultaneously involved, an unusual clinical presentation. An alternative explanation would be severe hypotension or impending circulatory collapse, rendering many target organs ischemic. He came in with marginal blood pressure (84/51 mmHg), and he was in heart failure (HF) (BNP 674 ng/ml). Besides myocardial damage (CKMB 50.9 ng/ml, troponin-I 10.8 ng/ml), there was liver injury (AST 322 IU/L and ALT 249 IU/L), both of which can result from hypotension or biventricular HF. Shock (total circulatory collapse) can cause multi-organ damage, including heart, brain, lung, liver, kidney, and even muscle (rhabdomyolysis).
As noticed, d-Dimer is elevated (2490 ng/ml). It is the final product of plasmin-mediated degradation of fibrin-rich thrombi. However, d-Dimer can become elevated in several chest emergencies, such as ACS, venous thromboembolism (including pulmonary embolism), acute aortic dissection, and circulatory instability, e.g., HF and cardiogenic shock. Hyponatremia (Na 113 mEq/dl) may be dilutional in the preset case due to HF or hypovolemia after diarrhea and vomiting. Fortunately, he was mentally clear on admission and had no associated hypokalemia. To correct hyponatremia, in this case, is a technical challenge. Given the significantly compromised left ventricular function (EF 32.6%), the care team needs to select intravenous fluid and titrate its rate of administration carefully,
With diffuse low ECG voltage and marginal blood pressure, unless otherwise explained (e.g., obesity, COPD, etc.), the differential diagnosis should consider pericardial effusion with impending acute cardiac tamponade by immediately ordering echocardiography after stabilizing the vital signs. Interestingly, the heart rate in sinus rhythm is relatively slow (61-66/min) when first seen. Under the clinical context, it might be related to vagotonia associated with inferior MI, acute cardiac tamponade, beta-blocker therapy, or a preexisting sinus nodal dysfunction. The fact that the patient suddenly developed VF and cardiac arrest is harmonious with all the above suspected clinical scenarios (i.e., ACS and pericardial effusion with acute cardiac tamponade).
To all surprise, no occlusive lesions exist in any coronary artery. With a history of recent gastroenteritis (watery diarrhea with nausea and vomiting), it is reasonable to entertain the diagnosis of FM. Although nonspecific, lack of leukocytosis (WBC 4800) associated with elevated CRP (2.66) is in line with a viral infection. Differential diagnosis should include stress-induced cardiomyopathy (the so-called Takotsubo disease), thyrotoxicosis, and peripartum cardiomyopathy if the patient is a younger female. Nevertheless, acute viral mLackyocarditis remains the most likely diagnosis because of the preceding history of gastroenteritis and his response to antiviral therapy.
The ECG in patients with acute myocarditis varies significantly among individuals. It can be normal or display a variety of abnormalities, none of which are pathognomonic. The most common ECG abnormality in myocarditis is sinus tachycardia with nonspecific ST/T-wave changes. Others include:
However, the sensitivity of ECG for the diagnosis of myocarditis is low, estimated at < 50% with unknown specificity. Diffuse low voltage is caused by edema of the ventricular wall, pulmonary edema, and peripheral edema. Intriguingly, the morphology of ST-segment elevation can be either convex (transmural myocardial injury), mimicking acute STEMI, or concave, resembling nonischemic etiologies such as early repolarization and acute pericarditis. As such, it is worth knowing that an ECG is not required to diagnose myocarditis. Myocarditis should be suspected in symptomatic or asymptomatic patients with an unexplained elevation of cardiac biomarkers (e.g., troponin).
The clinical manifestations of myocarditis are highly variable. Like ECG, no sign, symptom, or constellation of clinical features is diagnostic of acute or subacute/chronic myocarditis. The patient can be asymptomatic or present with the following:
FM may need emergency mechanical circulatory support, such as extracorporeal membrane oxygenation (ECMO) or IABP, as in the present case. Severe, diffuse myocarditis can result in acute dilated cardiomyopathy.
Acute myocarditis is an acute inflammation of the myocardium produced by various causes, many of which are infectious (viruses, bacteria, protozoa, fungi, etc.) In developed countries, viral infection is the most frequently presumed cause of myocarditis. Viruses can induce myocarditis through a direct myocardial injury (cardiotropic and vasculotropic viruses) or trigger abnormal immune system hyperactivity against the myocardium (lymphotropic viruses). In the latter, viruses indirectly cause myocarditis by triggering a cytokine storm or by activating self-reactive T-cell response due to molecular mimicry between viral and cardiac antigens. Other causes include exposure to toxic agents (e.g., alcohol, cocaine, radiation, etc.) or drugs (e.g., new anticancer class of immune checkpoint inhibitors), hypersensitivity reactions, and systemic and autoimmune disorders such as Celiac disease, collagen-vascular diseases, hypereosinophilia, sarcoidosis, giant cell myocarditis, thyrotoxicosis, etc.).
Endomyocardial biopsy (EMB) remains the gold standard for diagnosing myocarditis histologically. However, many suspected patients are clinically not considered candidates, and the yield is generally low due to frequently inadequate sampling. EMB examination is diagnostic for some specific causes of myocarditis, like giant cell myocarditis, sarcoidosis, and other autoimmune and infectious causes. However, the viral culture of myocardial samples for viruses is often unsuccessful. Cardiac magnetic resonance imaging (MRI) and Late gadolinium enhancement (LGE) help manage patients with myocarditis. In patients with “clinically suspected” myocarditis, MRI can detect interstitial edema during acute inflammation and later for monitoring disease progression, and LGE can assess subepicardial or mid-myocardial fibrosis in severe myocarditis cases with late arrhythmia complications.
In the future, prospective studies might be directed toward determining and validating the role of viral genome detection in the heart in diagnosing and managing inflammatory cardiomyopathy.
Oseltamivir (Tamiflu) is one of the neuraminidase inhibitors that can halt viral replication and disease progression.* Clinical experience with this drug therapy (e.g., 75 mg twice daily for five days in adults) for influenza (A and B) infection has been excellent. Therefore, early empiric oseltamivir therapy should be initiated when myocarditis is suspected, even when rapid viral diagnostic tests are negative. Lastly, immunosuppressive therapy might be necessary for chronic myocarditis with persistent inflammation. Overall, some patients will recover without residual myocardial injury, whereas others develop dilated cardiomyopathy.
*Paxlovid (nirmatrelvir and ritonavir co-packaged) is the first FDA-approved oral antiviral drug for treating mild-to-moderate COVID-19 infection in adults who are at high risk for progression to severe complications, including HF, FM, respiratory failure, and death.
Keywords:
acute coronary syndrome, acute myocarditis, cardiomyopathy,cardiogenic shock
UpToDate:
Myocarditis: Causes and pathogenesis
Clinical manifestations and diagnosis of myocarditis in adults
Buttà C et al. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: A comprehensive review. Ann Noninvasive Electrocardiol. 2020;25:e12726;doi.org/10.1111/anec.12726
Tschöpe C et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021 18:169–93.

This 92-year-old man complained of progressive dyspnea with PND, productive cough with whitish sputum, and bilateral leg edema for the past few weeks. He was
A 52-year-old man was admitted because of progressive dyspnea on exertion for one week. In the past, he had chronic obstructive pulmonary disease (COPD), alcoholic
A 26-year-old Taiwanese man noted weakness in both lower legs and could not stand upon waking up. At the same time, he experienced upper back
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.