Case 15
A 20-year-old man awakened from sleep with severe chest tightness and shortness of breath (SOB). Simultaneously, he felt dizzy and nauseous and fainted with crutches
A 66-year-old woman was admitted with chest tightness and intermittent palpitations at rest for one week. Past medical history is significant for hypertension and chronic obstructive lung disease (COPD). Reportedly, she underwent lobectomy (two-thirds of lobes) for a right lung tumor about 30 years prior. She also had recently documented paroxysmal atrial flutter (AFL)/fibrillation (AF). She had shortness of breath with mild exertion and had a cough with yellowish sputum but denied having fever, chills, and hemoptysis. She was in apparent respiratory distress; BT/PR/RR measured 36.6℃, 120/min, irregular, and 24/min, respectively. BP measured 130/82mmHg. Physical findings were positive for JVD of 11 cm H20, bilateral 2+ leg edema, a displaced PMI at LAAL in the left 6th intercostal space, an RV lift, and a GrII/IV systolic murmur at the apical area; the inspiratory phase was prolonged with wheezing sounds, and there were crackles at the right posterior base. ECG revealed diffuse low voltage, RV enlargement, and AFL at a rate of 250/min, with periodic A-V Wenchbach accounting for ventricular irregularity. Chest X-ray showed findings suggesting LV, LA, and RA enlargement, along with pulmonary hypertension (PH), congestion, and possible right lower lobe pneumonia. Significant laboratory findings included ABG: pH 7.458, PO2 62.5 mmHg, PCO2 27.6 mmHg, HCO3 19.1 mmol/L, BE -3.2 mmol/L, Hb 14.8, gm%, WBC 14.75 10×3/uL (85% neutrophil), Plt 180 10×3, CRP 4.2 (N <0.6) mg/dL, BUN 24 mg/dL, Cr 0.92 mg/dL, Na 138 mEq/L, K 3.8 mEq/L, SGOT 83 (N 12-45) U/L, CK MB 39.6 (N <25) ng/mL, and troponin-I 0.09 (N <0.05) ng/mL. TSH and Free T4 were within normal range (1.8 [N 0.27-4.2] uIU/mL and 1.47 [N 0.93-1.71] ng/dL, respectively). In light of possible pneumonia, the team immediately started antibiotic therapy (amoxicillin and clavulanic acid) after taking sputum for culture while managing heart failure (HF). Intravenous amiodarone bolus was given, followed by a maintenance drip to slow the ventricular rate of AFL (ECG 2). Since AFL was likely present for several days, the care team used oral rivaroxaban 15 mg daily for anticoagulation to prevent embolic stroke and ordered a transesophageal echocardiography (TEE). The patient was in AFL with 2 :1 A-V conduction during the TEE examination. It disclosed normal LV wall thickness with mildly impaired apical wall motion; LV systolic function was well preserved. However, E/A was reversed, consistent with mild LV diastolic dysfunction. Of note, LA was only slightly dilated (39.15 [N 39.0] mm), and there was no thrombus in LA and its appendage. RA and RV were enlarged with moderate TR; the estimated RA pressure was 10-15 (N 0-5) mmHg, and PA systolic pressure was 67-72 9 (N 30-35) mmHg. On the fourth day of rivaroxaban therapy, the care team performed a QRS-synchronized low-energy (50 joules) electrical cardioversion, successfully converting AFL to sinus rhythm (ECG 3). The patient tolerated the procedure well. She continued to improve (Chest X-ray 2). Subsequent persantin-thallium scan demonstrated moderate ischemia in the basal anterior and lateral walls. Accordingly, the care team instituted an anti-ischemic regimen (long-acting nitrates). She remained in a sinus rhythm before discharge.
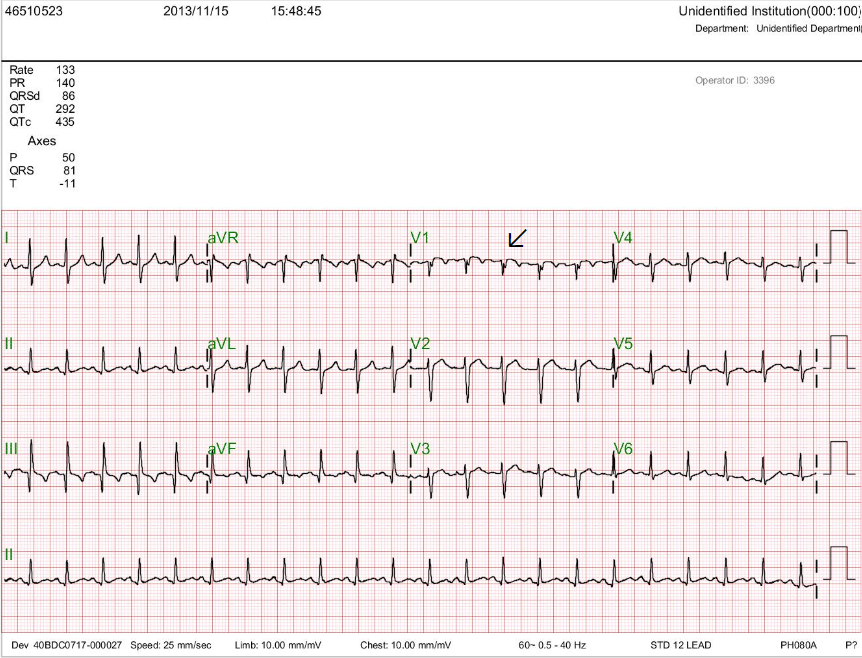
Diffuse low voltage
Atrial flutter (AFL) or atrial tachycardia (atrial rate 250/min and ventricular rate 237/mi) with periodic Wenckebach (the arrow points to the non-conducted atrial activity that is buried within the QRS complex)
S1S2S3 pattern with left axis deviation (<-30。)
Diffuse nonspecific T wave changes.
Tall R wave in V1 and S wave in V5 and V6 with equal R/S in V2-V6 suggestive of RVH or biventricular enlargement
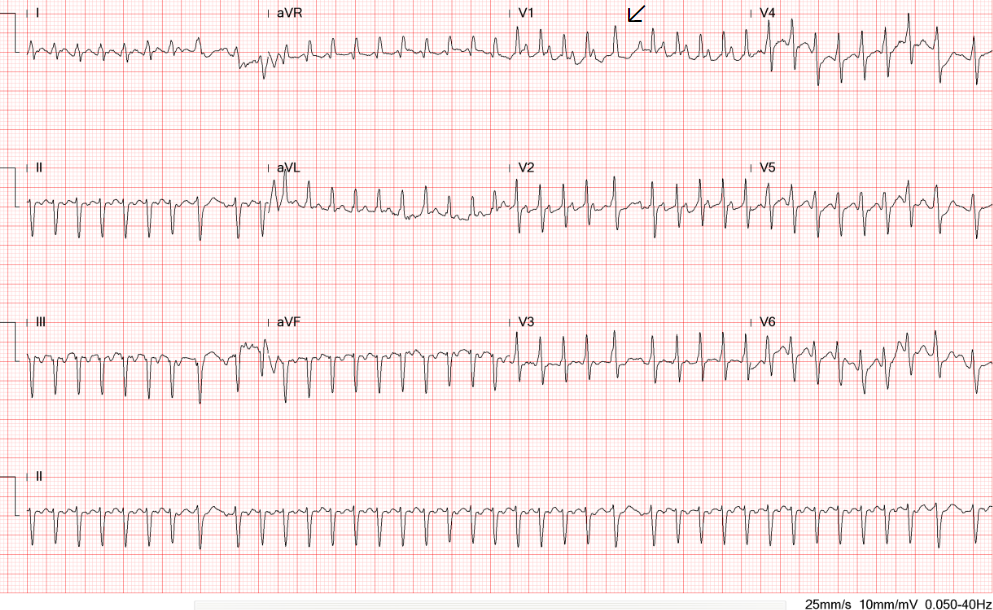
AFL (slowed) with a 2:1 A-V block (atrial rate 226/min, ventricular rate 113/min)
Right axis deviation (+109。) with qR in V1 and tall R wave in V1, and equalization of R/S in V2-V6 suggestive of RVH or biventricular enlargement
P wave morphology suggestive of LA enlargement or intra-atrial conduction delay
Compared to the ECG on admission, the QRSd increased from 99 msec to124 msec, presumably due to the amiodarone effect
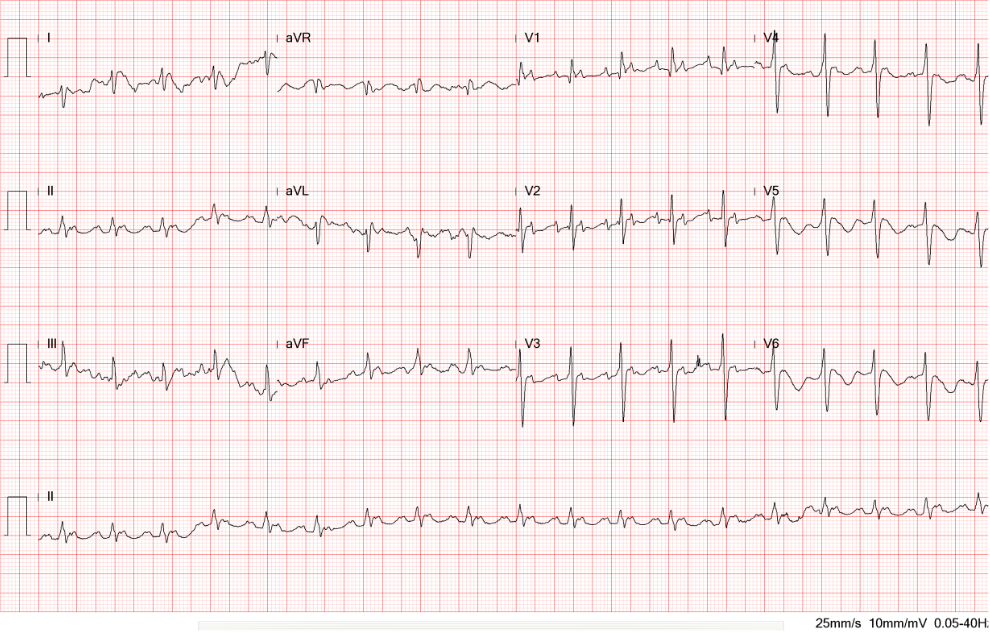
Sinus rhythm at 69/min
LA enlargement based on the P wave morphology in leads II and V1
Right axis deviation (+129。) with qR in V1 and tall R wave in V1, and equalization of R/S in V2-V6 suggestive of RVH or biventricular enlargement
q (up to 40 msec) in V1 and V2, r/o an old septal infarction
Compared to the ECG on admission, the QRSd increased from 99 msec to 126 msec (RBBB pattern) presumably due to the amiodarone effect
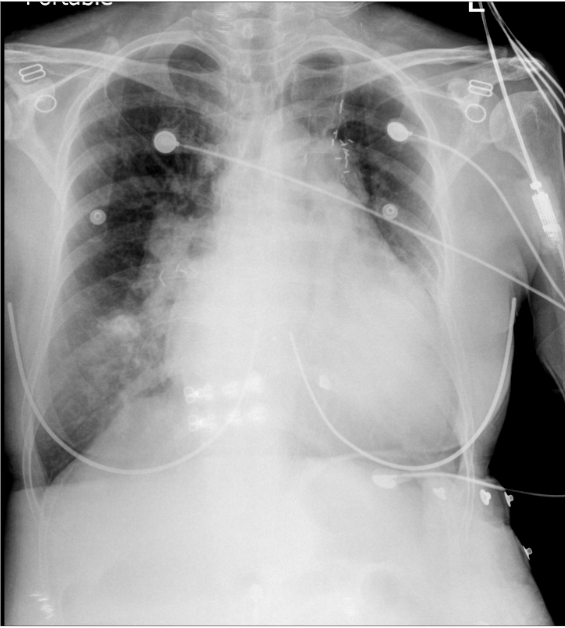
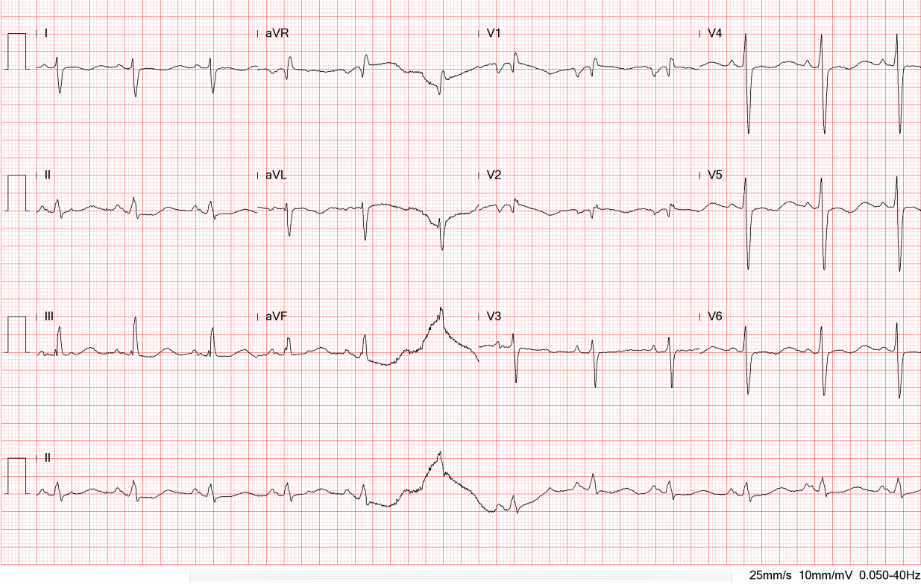
Cardiomegaly with LV, LA, and RAenlargement
Prominent pulmonary arteries (bilateral) suggestive of pulmonary hypertension (PH).
Pulmonary congestion with patchy and nodular density at the right lower lobe. Pneumonia cannot be ruled out.
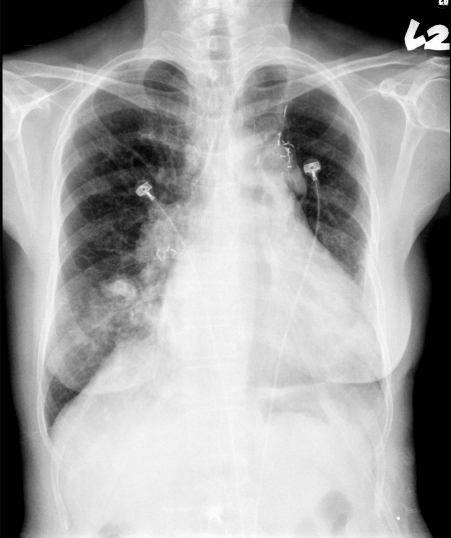
Improvement of heart failure and resolution of pneumonia
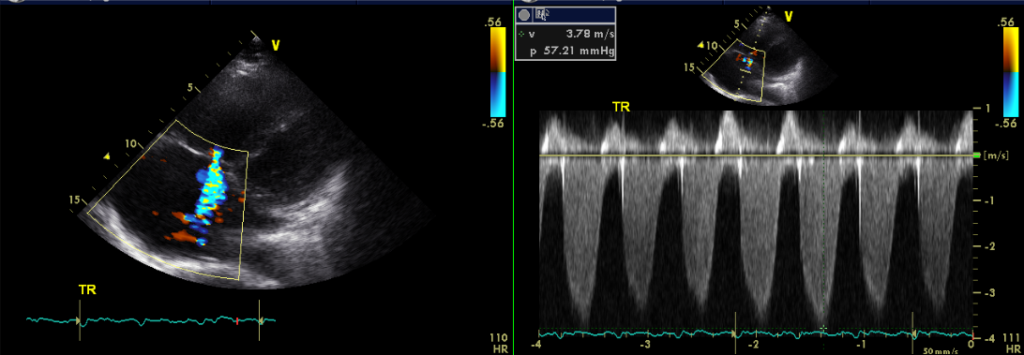
AFL with 2 :1 A-V conduction
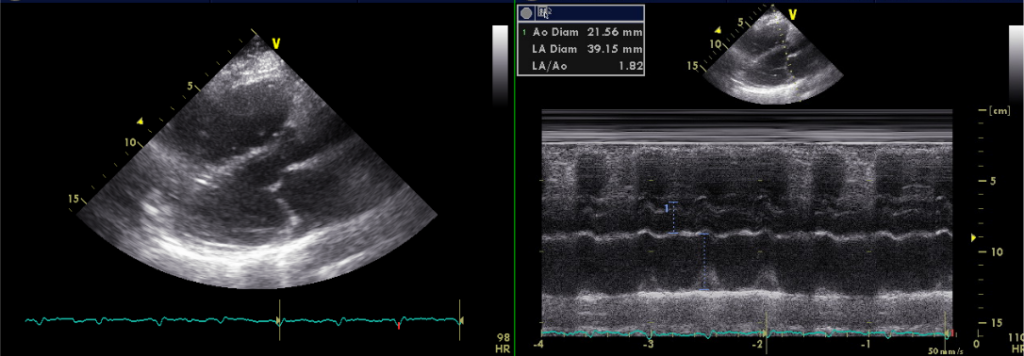
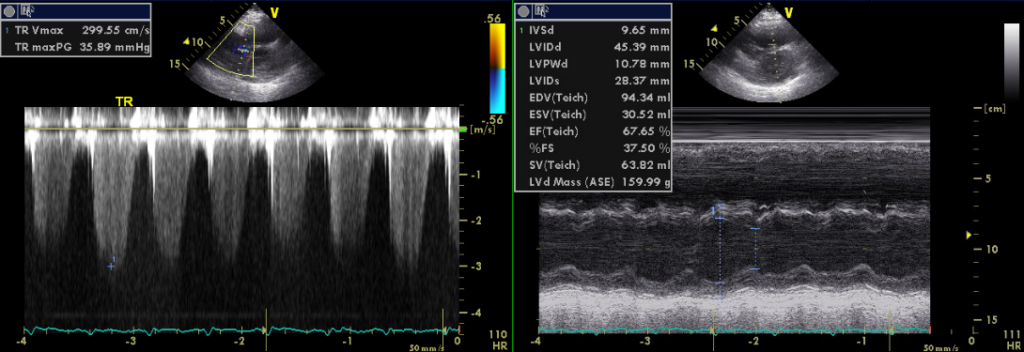
Normal LV wall thickness with mildly impaired apical wall motion with preserved LV systolic function
LA 39.15 mm
Sclerosing change of aortic valve without AS or AR
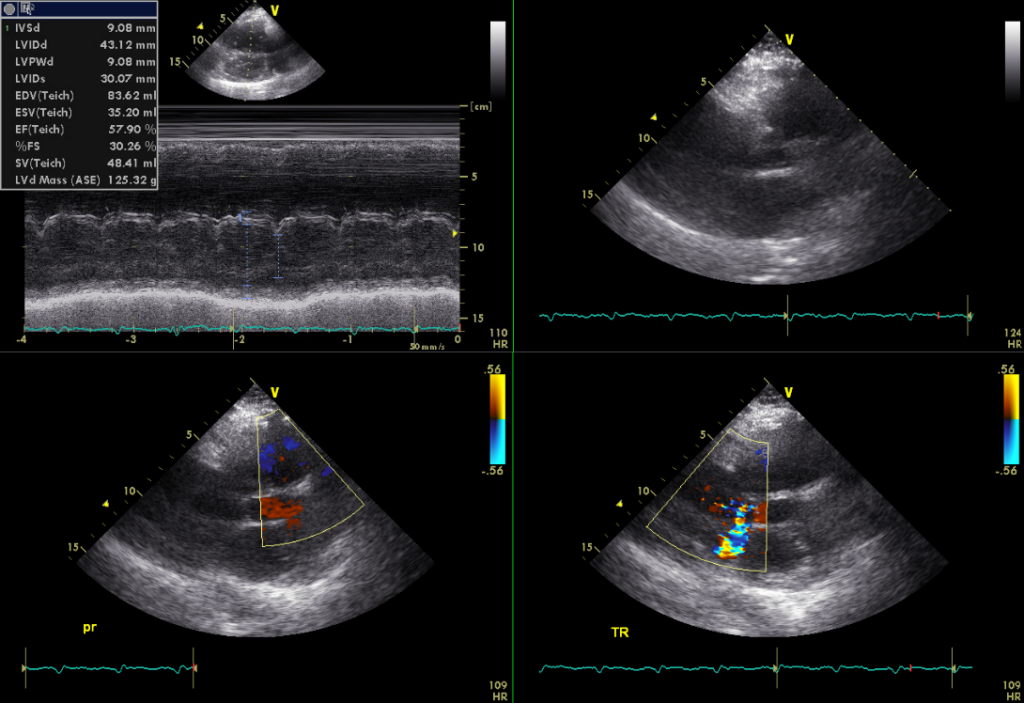
Dilated RA and RV with mild MR, Moderate TR, and trivial PR
Estimated RA pressure: 10-15 mmHg and PA systolic pressure: 67-72 mmHg
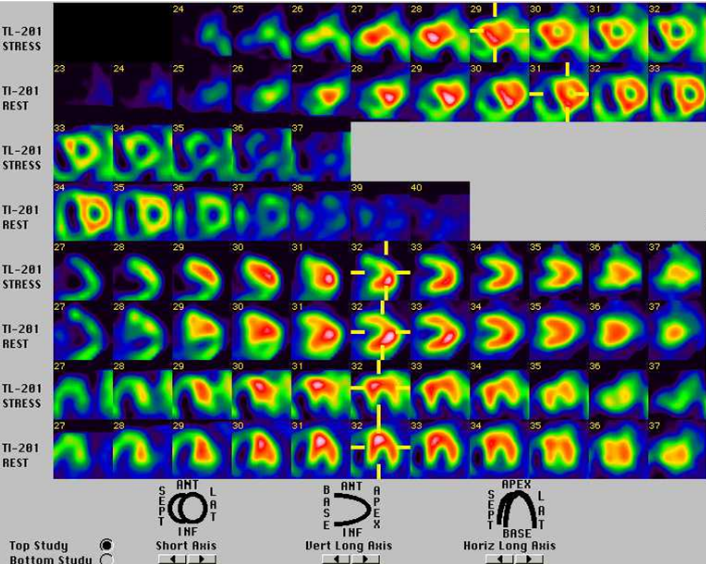
Moderate ischemia in the basal anterior wall and basal lateral wall
The automated reading system misinterprets the initial ECG rhythm as AF due to the irregularity of the ventricular response. As we identify atrial activities (leads III and V1) to be regular at 250/min, the correct reading of the rhythm is AFL (average 300/min, range 240-340/min). Alternatively, it is a form of “fast” focal (ectopic) atrial tachycardia. Leads III and V1 best illustrate progressive A-V (PR) interval prolongation before failure of A-V conduction (a non-conducted atrial activity; A-V Wenchebach), accounting for the ventricular response’s irregularity. The tall R wave in V1 and prominent S wave in V5 and V6 offer the diagnosis of RVH with severe clockwise rotation or biventricular enlargement. Together with the S1S2S3 pattern, these ECG findings resonate with significant COPD with PH; However, the ECG diagnosis of biventricular hypertrophy (i.e., Katz-Wachtel phenomenon*) might not be appropriate in diffuse low-voltage.
TEE, originally ordered to rule out LA thrombus, has provided critical information concerning this patient’s primary clinical problem: right-sided HF due to her longstanding COPD with PH (PH group III)** might be the main issue. The estimated RA pressure is 10-15 (N 0-5) mmHg, and pulmonary artery systolic pressure is 67-72 mmHg (N less than 40 mmHg by TEE). Surprisingly, despite mildly impaired apical wall motion, LA is only slightly dilated, and LV is normal in size with normal wall thickness and preserved systolic function. In retrospect, the chest X-ray impression of LA and LV enlargement is likely caused by COPD and a hyperinflated right lung due to a previous lobectomy (2/3 of lobes), shifting the heart more to the left. As such, differentiation between typical and atypical AFL based on the flutter wave morphology*** might not be proper because of the displaced anatomy of the heart relative to the torso.
In real-world practice, it is common to encounter COPD and left-sided HF. Under the circumstances, left-sided HF is usually related to some form of cardiomyopathy (hypertensive or ischemic, i.e., atherosclerotic coronary artery disease [CHD] with prior MIs, etc.). On the other hand, COPD often has a component of emphysema, chronic asthma, or bronchitis. COPD often leads to underestimating the severity of left-sided HF on Chest X-rays. The two conditions existing together can exacerbate one another. COPD can cause low oxygen levels in the blood, placing additional stress on the heart and worsening symptoms of left-sided HF.
Furthermore, treating one condition may exacerbate the other. For example, β-blockers are widely prescribed in the treatment of CHD, whereas β-agonists represent a cornerstone of COPD treatment. Beta-agonists and anti-muscarinic agents drugs favoring COPD may worsen CHD, provoking myocardial ischemia and cardiac arrhythmias. Gathering all detailed information and making accurate clinical diagnoses are essential in forming a suitable treatment strategy.
ABG confirmed significant hypoxia PO2 (62.5 mmHg) with compensatory respiratory alkalosis (pH 7.458, pCO2 27.6 mmHg). Hypoxia can further cause the constriction of the pulmonary arterioles, worsening pulmonary hypertension. Higher PaCO2 values and more elevated PA systolic pressure significantly raise the prevalence of atrial arrhythmias. Aside from starting treatment for right-sided HF and possible pneumonia, the care team realized the urgency of controlling AFL with a rapid ventricular response; AFL/AF and right-sided or left-sided HF are mutually causative. Failure to adequately control these arrhythmias will lead to a vicious cycle of hemodynamic derangement. Since AFL is likely to be present for more than 48 hours, the care team used rivaroxaban therapy to prevent stroke and simultaneously infused intravenous digoxin and amiodarone (bolus followed by drip) to slow the ventricular rate in preparation for TEE and electrical cardioversion.
The so-called novel oral anticoagulants have a rapid onset, fixed dosing, and no required routine monitoring. Rivaroxaban is a factor Xa inhibitor. After oral ingestion, it is rapidly absorbed, achieving maximum concentration in 2–4 hours. Its administration as short as 4 hours before cardioversion can be effective and safe. In the present case, the care team waited three days after rivaroxaban administration, and the ventricular rate of AFL slowed to 113/min (2:1 A-V block) before electrical cardioversion. This approach has rid of the three to four weeks of therapeutic vitamin K-antagonist (warfarin) anticoagulation required before cardioversion, a drawback of the conventional method. However, a recent retrospective analysis has revealed that drug-drug interaction between amiodarone and oral factor Xa inhibitors increases the risk of severe bleeding and bleeding-related death. In this situation, it is suggested that a proton pump inhibitor therapy might help reduce the incidence of gastrointestinal bleeding complications.
Intravenous amiodarone is advantageous in this clinical setting due to its relatively neutral effect on cardiac contractility. However, the long-term use of amiodarone may not be an option. Amiodarone interacts with digoxin by interfering with its renal clearance, increasing the risk of digoxin toxicity. On long-term oral therapy, amiodarone also exerts many severe side effects, including visual disturbance, hyperthyroidism, liver toxicity, pulmonary toxicity (hypersensitivity pneumonitis or interstitial/alveolar pneumonitis), etc.
Intriguingly, TEE and persantin-thallium scan results indicate that the patient might also have atherosclerotic CHD. For over a decade, investigators have suggested the increased risks for CHD in COPD patients. Besides smoking, chronic hypoxia, and hypercapnia can lead to widespread inflammatory changes as evidenced by elevated TNF-alpha, interleukin IL6, fibrinogen, and C-reactive protein, etc., which, in turn, along with the aging process, accelerate atherosclerosis of the endothelium. Many investigators have thus advocated early intervention in COPD patients, such as modifying their lifestyle and lipid profiles, and adding an antiischemic regimen if necessary.
The systemic inflammatory effects of COPD also affect the atrium, promoting matrix metalloproteinase expression (an essential molecular for regulating atrial structural remodeling). Together with the aging process, it accelerates degenerative changes in the atrium prone to developing atrial arrhythmias. If the patient is stable enough, catheter ablation is a first-line approach to managing typical AFL (cavotricuspid isthmus (CTI)-dependent) *** in patients with PH. However, because of the RA and tricuspid annular dilation, CTI ablation is often technically challenging. In addition, due to extensive atrial remodeling, focal atrial tachycardias, non-CTI-dependent atypical AFL, and multiple AFL circuits are common. It is technically even more difficult for AF ablation as non-pulmonary venin AF triggers such as the superior vena cava, LA free wall, crista terminalis, coronary sinus ostium, ligament of Marshall, etc., are more prevalent in patients with COPD.
When performing left-sided ablation, one should avoid the iatrogenic creation of an atrial septal defect during transseptal puncture. With high RA pressure (10-15 [N <5] mmHg), the atrial septal defect could engender a right-to-left shunt, producing chronic hypoxemia. Thus, radiofrequency ablation might be preferable to cryoablation under this clinical scenario, as the latter requires a larger transseptal catheter sheath.
Lastly, in accessing RVH using chest X-rays, a lateral view is required (not done). There is a slight elevation of cardiac enzymes without serial follow-up results; a small MI cannot be excluded. Moreover, with PA pressure over 50 mmHg indicating the chronicity of PH, an acute pulmonary embolism on top of chronic PH cannot be ruled out (d-Dimer not done); a chest CT scan might be necessary.
*Katz-Wachtel phenomenon: Tall biphasic QRS (tall R and deep S waves >50 mm) in the precordial leads (usually V2-V5) typically associated with biventricular hypertrophy in patients with congenital heart disease (e.g., VSD).
**Classification of pulmonary hypertension (PH)
Group 1: Pulmonary arterial hypertension (PAH)
Group 2: PH due to left-sided heart disease
Group 3: PH due to lung disease or hypoxia
Group 4: PH due to pulmonary artery obstructions, including chronic thromboembolic PH
Group 5: PH with unknown or multiple causes
***Typical atrial flutter (AFL) involves a macroreentrant circuit traversing the cavotricuspid isthmus (CTI), the region of RA tissue between the orifice of the inferior vena cava and the tricuspid valve annulus (counterclockwise or clockwise rotation). If the CTI is not involved, it is called “atypical” AFL. This type of AFL can affect any region of RA or LA. In typical AFL, the flutter waves in the inferior leads (II, III, and aVF) and lead V1 are often disconcordant, whereas in atypical AFL, flutter waves are concordant. Catheter ablation is much easier in typical than in atypical AFL.
Keywords:
atrial flutter, amiodarone, novel oral anticoagulants, pulmonary hypertension
UpToDate:
Overview of atrial flutter
Clinical features and diagnosis of pulmonary hypertension of unclear etiology in adults
Cardioversion for specific arrhythmias
Morgan AD et al. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Therapeutic Advances in Respiratory Disease 2018;12: 1–16.
Ray WA et al. Risk of bleeding-related hospitalizations during use of amiodarone with apixaban or rivaroxaban in patients with atrial fibrillation: a retrospective cohort study. Ann Intern Med 2023;176(6):769-778.

A 20-year-old man awakened from sleep with severe chest tightness and shortness of breath (SOB). Simultaneously, he felt dizzy and nauseous and fainted with crutches
A 72-year-old woman, who had been in the hospital for treatment of right leg cellulitis for two days, suddenly developed increasing SOB with worsening heart

An 88-year-old woman complained of a loss of appetite, generalized weakness for two weeks, and increasing SOB for five days. She had chronic heart failure
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.