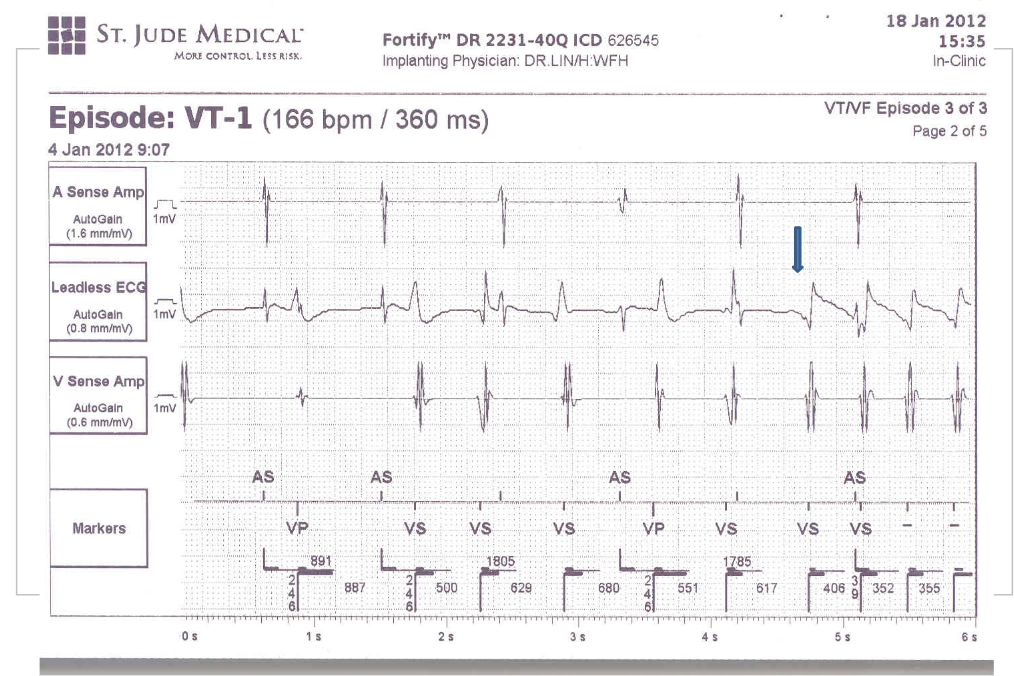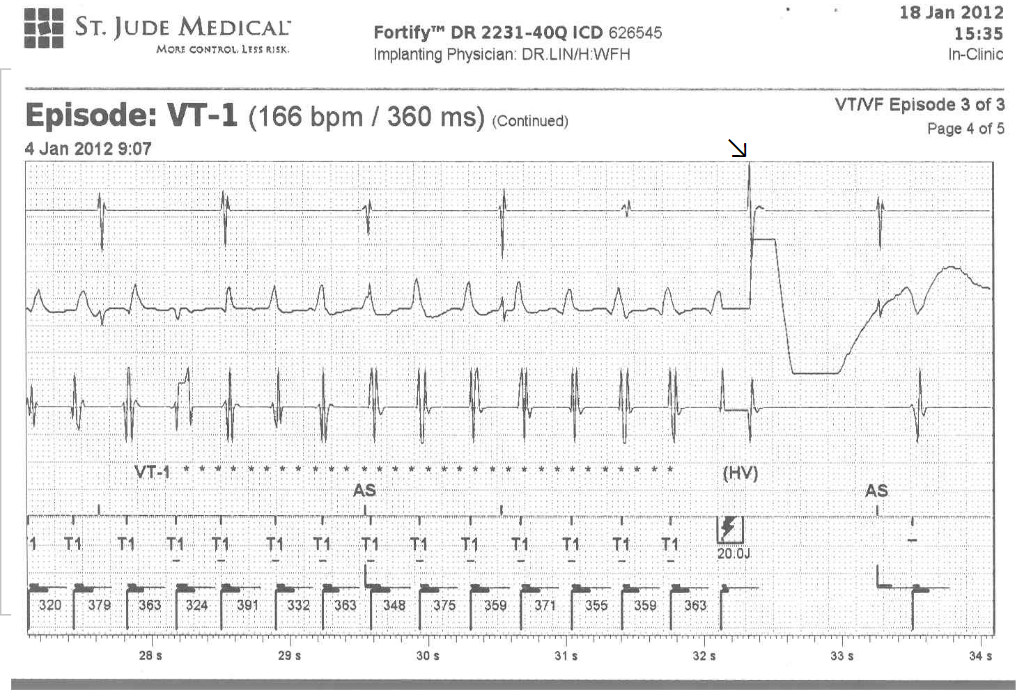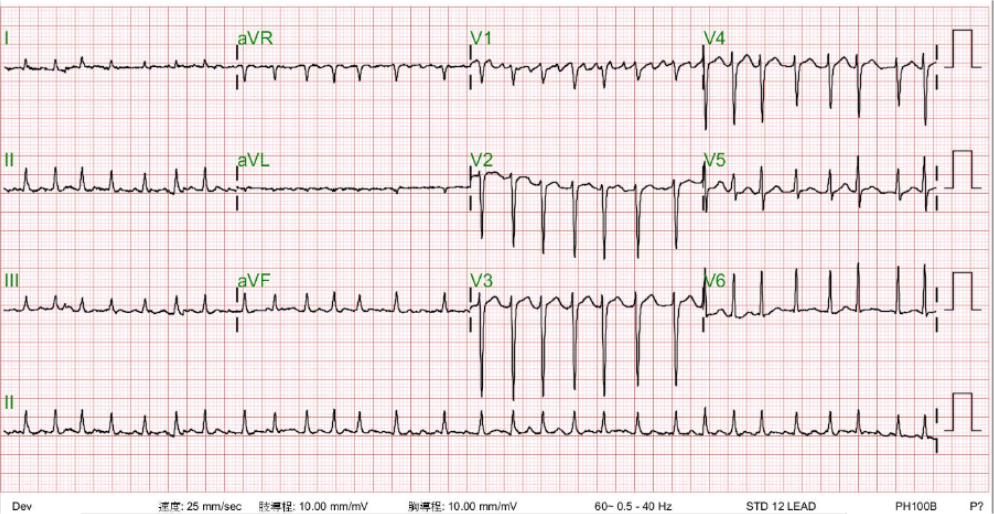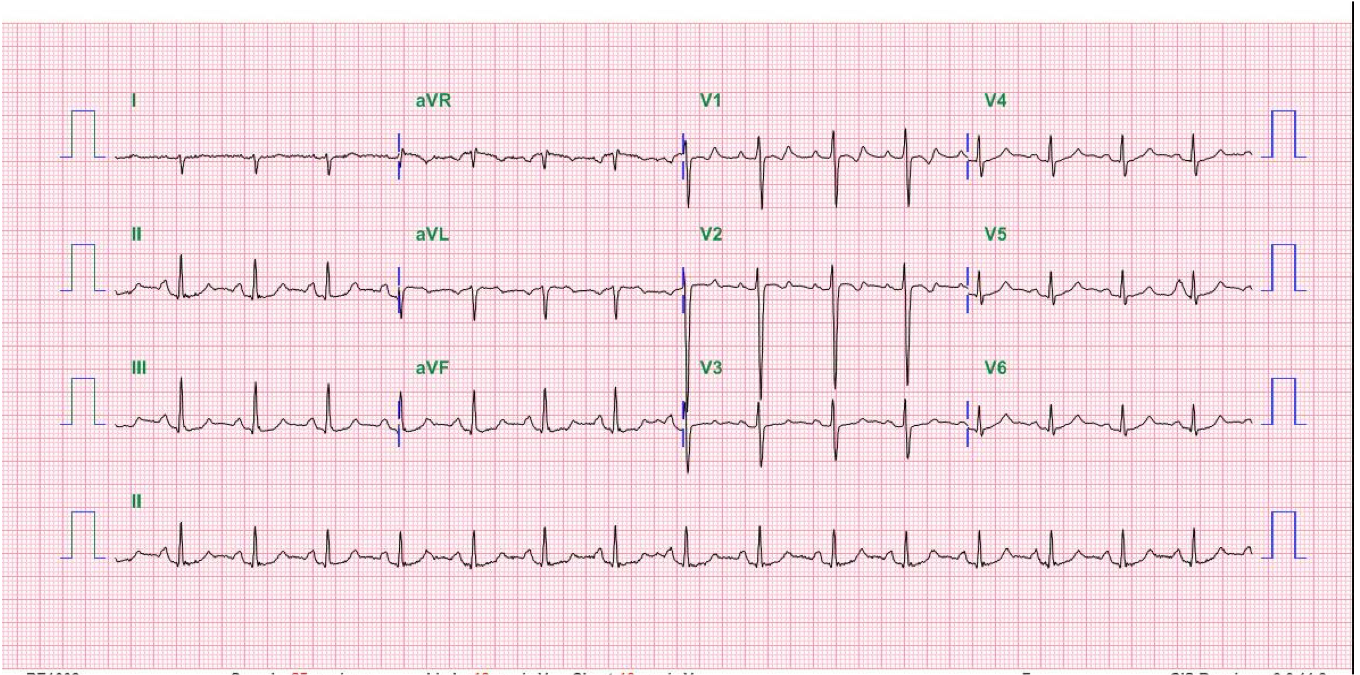
Case 32
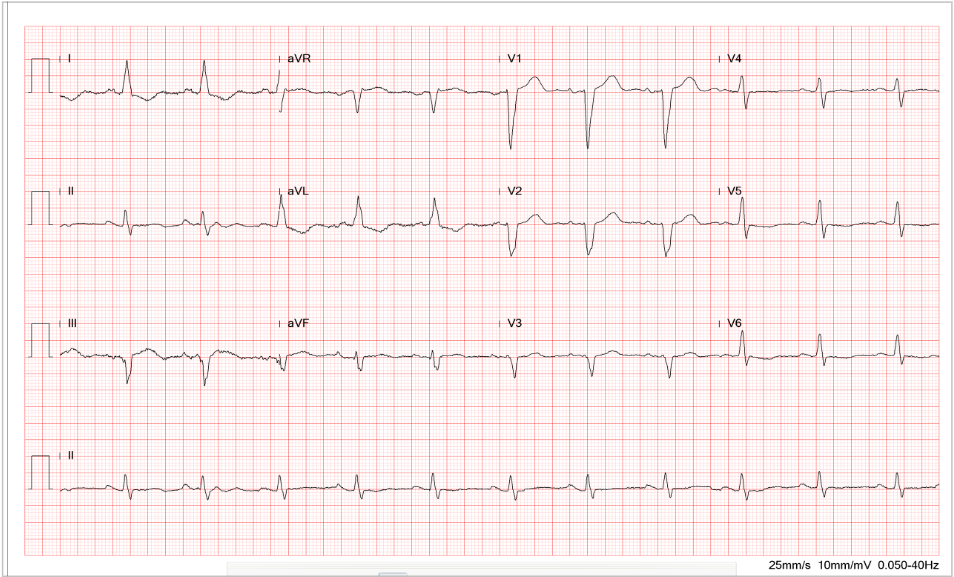
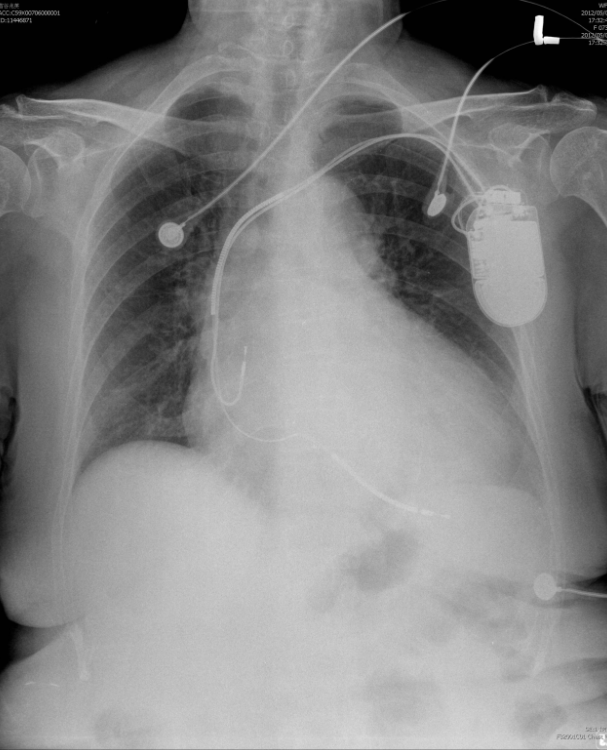
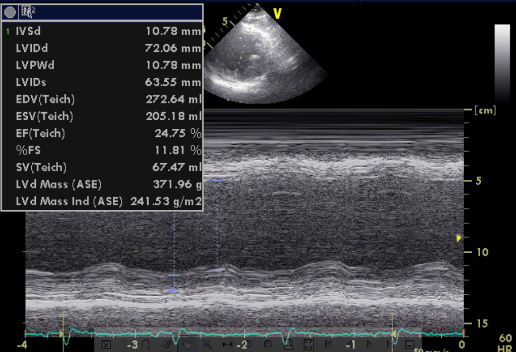
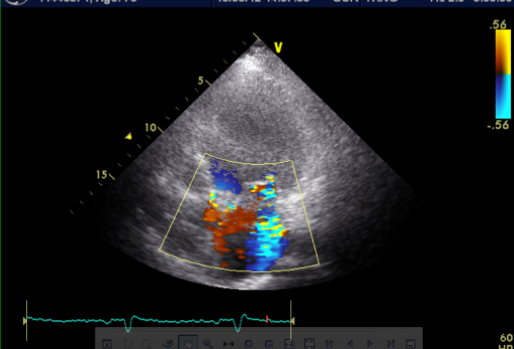
A 52-year-old man was admitted because of progressive dyspnea on exertion for one week. In the past, he had chronic obstructive pulmonary disease (COPD), alcoholic liver cirrhosis with minimal esophageal varices, gastroesophageal reflux, and duodenal ulcer with bleeding but no hypertension, diabetes mellitus, or atherosclerotic coronary heart disease (CHD). He was one pack a day smoker and a daily drinker for at least 25 years.