
Case 14
A 30-year-old woman, a professional cook, suddenly experienced retrosternal chest tightness after dinner, which increased in intensity without radiation, lasting for 2 hours.
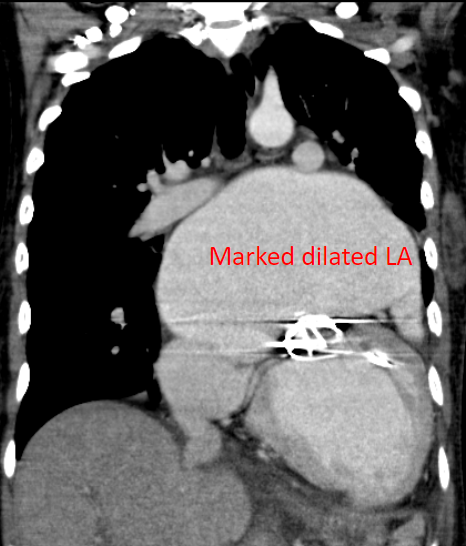
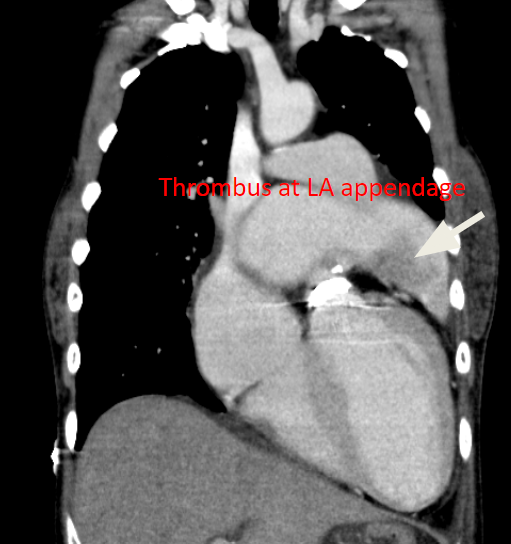
A 35-year-old woman with Marfan syndrome presented with increasing dyspnea, fever, productive cough, and chest tightness for one day. PMH was significant in that she had multiple surgical procedures, i.e., bilateral lens fixation 15 years ago, surgical correction of anomalous left coronary artery (LCA) and mitral valve (MV) replacement 11 years ago, and Bentall procedure (composite graft replacement of the aortic valve, aortic root, and ascending aorta and re-implantation of coronary arteries into the graft) 9 years ago. She complained of no chills, chest pain, palpitation, orthopnea, PND, and leg edema. She had bronchitis three weeks before admission and received antibiotic therapy. A local physician first saw her and, based on the elevation of Tn-I, transferred her to Emergency Department under the impression of myocarditis vs. acute NSTEMI. On arrival, she appeared exhausted but in no acute distress. BT was 38.1°C, PR 84/min, BP 94/65 mmHg and RR 14/min. There was no leukocytosis (6900/mL), but the cardiac enzyme Tn-I remained elevated at 1.5 [N: <0.5] ng/mL. Serial blood cultures were negative. Chest X-ray showed cardiomegaly with a markedly enlarged LA, prominent PA suggestive of pulmonary hypertension, mild pulmonary congestion, possible pneumonia, and prosthetic (mechanical) mitral and aortic valves. Cardiac CT revealed the dilated LA with a thrombus in the appendage despite an INR of 2.06. Echocardiography revealed LA 59 (N:19-40) mm; LV 59 (N:19-40) mm with LV EF 41%, mild TR, and regional wall motion abnormality with severe hypokinesis of the anteroseptal, mid-to apical septal segment; PA pressure (RVSP) was 29 (N < 30) mmHg, the aortic valvular area was estimated to be 2.03 (N 3.0-4.0) cm2, and the mitral valvular area 1.51 (N 4.0-6.0) cm2. After a course of antibiotic therapy for pneumonia, her condition improved. Subsequent cardiac catheterization with coronary angiography revealed patent aortic graft and patent grafts to the right coronary artery (RCA) and LCA (for previously anomalous origin of LCA) running from the right lateral aspect of ascending aorta with a course between the aorta and the markedly dilated LA; the latter finding was thought to be the culprit of NSTEMI on this admission. Accordingly, surgical consultation was obtained.

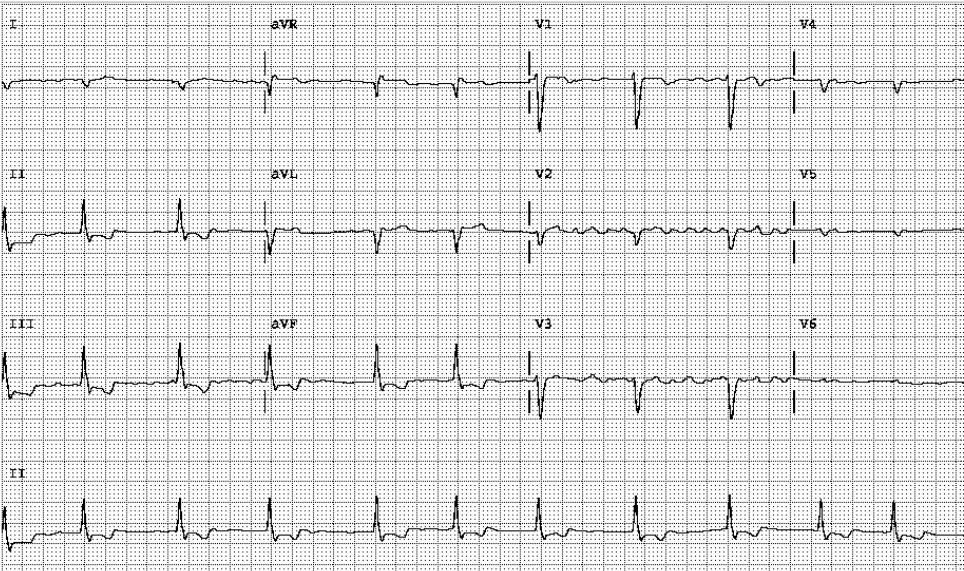
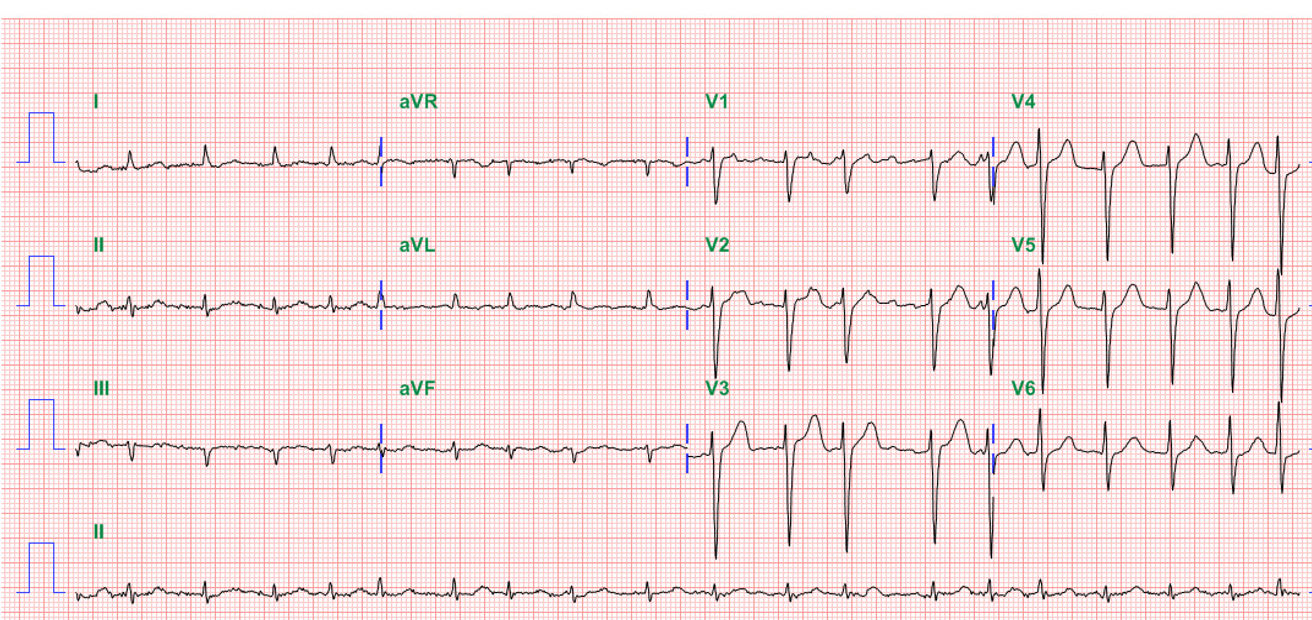
AF with a ventricular rate of 78/min.
QS pattern in I and aVL c/w high lateral MI of undetermined age, probably old
Right AD (+120°) with slow progression of R in precordial leads (clockwise rotation) likely due to RVH
LV enlargement and biventricular enlargement cannot be excluded
ST elevation (1mm) in aVR (arrow) and ST depression with T inversion in II, III, aVF, and V6 denoting extensive subendocardial ischemia/infarction (NSTEMI)
No evidence of RV involvement
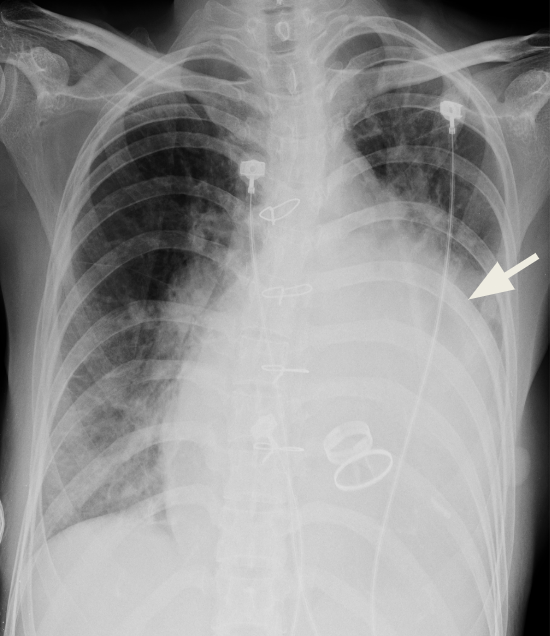
Cardiomegaly with a relatively small aortic knob.
Marked LA enlargement (i.e., widened carina, double density with a bulged cardiac border below left PA (arrow)
Likely also LV enlargement
Prominent PA suggestive of pulmonary hypertension
Infiltrates with haziness in bilateral lung fields suggestive of pulmonary congestion or pneumonitis
Prosthetic (mechanical) mitral and aortic valves.
Abnormal coronary artery origin is a rare disease with multiple variations. The most common anomaly is the left circumflex from the right sinus of Valsalva, followed by a single coronary artery from the left sinus of Valsalva, both coronary arteries from the right sinus of Valsalva, and the left anterior descending (LAD) artery from the right sinus of Valsalva. The subsequent course of the LCA between the aorta and pulmonary artery to the left ventricle is susceptible to compression, especially during exercise. Alternatively, it predisposes to blood flow obstruction if it runs an intramural course. In either situation, myocardial ischemia/infarction may ensue, resulting in MI and sudden cardiac death, especially in young athletes. The present case is further complicated by Marfan syndrome, an inherited connective tissue disorder affecting multiple organ systems (e.g., ocular, cardiovascular, musculoskeletal, lung, skin, and central nervous system).
The ECG findings are significant for AF, biventricular enlargement, an old high lateral wall infarction, and subendocardial ischemia/infarction pattern in the inferolateral wall. The old high lateral MI is likely caused by the previous “anomalous LCA.” With the elevation of cardiac enzymes (Tn-I 1.5 ng/mL), the subendocardial ischemia/infarction pattern in the inferolateral wall is a form of NSTEMI clinically presented as an acute coronary syndrome. Also, the reciprocal ST-segment elevation of the cavitary lead, aVR, might indicate a significant extent of myocardial ischemia*.
AF is age-related and rarely seen at a young age unless associated with LA enlargement due to structural heart disease, such as in the present case. Chronic pressure in the left atrium is the primary cause of LA enlargement, which accelerates tissue degeneration leading to the development of AF, which can lead to further LA enlargement (AF begets AF).
The patient appears not adequately anticoagulated (INR 2.06; INR recommended for prosthetic valve should be 2.5-3.5)**. LA is a posterior structure but centrally located. LA enlargement may compress the surrounding structures, including the esophagus, pulmonary veins, trachea, left main bronchus, middle and lower lobes of the right lung, inferior vena cava, recurrent laryngeal nerve, and thoracic vertebrae. In the present case, whether the LA enlargement (not enough to be called giant LA yet [>65 mm]) has affected the patent grafts to RCA and LCA in the present case remains to be determined. The care team recommended that the MV prosthesis be replaced after removing the thrombus and that atrial reduction surgery be considered because of the vast left atrium (59 mm).
*The significance of ST elevation in aVR is discussed in Case 3.
**Although for the aortic or pulmonic On-X valve (the newly developed bileaflet mechanical valve [pure pyrolytic carbon, devoid of silicon]), INR can be reduced to 1.5-2.0 after 2. 5 for the first three months, concomitant aspirin 75-100 mg daily is recommended. However, in patients with prior thromboembolism, AF, rheumatic mitral stenosis, or LV EF <35%, keeping INR at 2.5 remains advisable.
Keywords:
anticoagulation, atrial fibrillation,anomalous coronary artery, giant left atrium
UpToDate:
Congenital and pediatric coronary artery abnormalities
Genetics, clinical features, and diagnosis of Marfan syndrome and related disorders
El Magharaby A and Hajar R. Giant left atrium: A review. Heart Views

A 30-year-old woman, a professional cook, suddenly experienced retrosternal chest tightness after dinner, which increased in intensity without radiation, lasting for 2 hours.
An 84-year-old man with a known history of hypertension, COPD, diabetes, Parkinsonism, and old CVA (putamen infarction)* was noted to be delirious after five days
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.