Case 19
A 75-year-old man complained of worsening dyspnea with orthopnea for 2 hours. He had no chest pain or palpitations. He admitted dietary indiscretion and noted
A 52-year-old man was admitted because of progressive dyspnea on exertion for one week. In the past, he had chronic obstructive pulmonary disease (COPD), alcoholic liver cirrhosis with minimal esophageal varices, gastroesophageal reflux, and duodenal ulcer with bleeding but no hypertension, diabetes mellitus, or atherosclerotic coronary heart disease (CHD). He was one pack a day smoker and a daily drinker for at least 25 years. Current medications included theophylline anhydrous sustained-release microsphere capsules and tiotropium bromide (bronchodilator, a long-acting muscarinic antagonist) inhalation solution for COPD with chronic productive cough. A recent Flow-Volume curve showed mild obstructive ventilatory defect with a post-bronchodilator FEV1/FVC of 72%.* He was afebrile but in apparent SOB with PR 101/min, RR 20/min, and BP 106/64 mmHg. There was jugular vein engorgement at 11 cm H2O. PMI was invisible, but there was an RV lift at the left sternal border. S2 was loud in the pulmonic area, and a systolic murmur at the left lower sternal border increased in intensity with inspiration. Lungs were clear (no rales or wheeze). The liver was three-finger-breath palpable below the right costal margin, pulsatile. Leg edema was 3+ bilateral. Laboratory data included Hb 12.3 g/dl, Hct 39.3%, WBC 6860, Plt 218, PT 12.3, INR 1.22, D-dimer 655 (N <500) ng/ml**, BNP 113 (N <100 pg/mL), CKMB <0.1 (N <7.7 ng/mL), troponin <0.05 (N <0.05) ng/ml, Albumin 3.3 (N 3.4 to 5.5) g/dl, SGOT 118 (N 5 to 40) U/L, SGPT 86 (N 7 to 56) U/L, Alk-P 164 (N 20-140) U/L, r-GT 162 (N 0-30) U/L, BUN 13.7 mg/dl, Cr 1.2 mg/dl, Na 140 mEq/L, K 3.6 mEq/L, Amonia 67.84 (N 26–94) mcg/dL. The previous workup for viral hepatitis was negative. ECG showed sinus rhythm at 94/min, RA enlargement, and RVH with strain. Chest X-ray revealed COPD, RA, and RV enlargement (lateral view) and prominent pulmonic trunks, indicating severe pulmonary hypertension (PH). Echocardiography, on the other hand, besides dilated RA and RV, severe PH with significant TR, also showed LA enlargement (4.3 cm, LA/AO ratio 1.54) and good LV contractility with paradoxical septal wall motion and asymmetric septal hypertrophy. With a slight elevation of D-dimer and possible left-sided cardiomyopathy, the care team performed cardiac catheterization with angiograms in an attempt to clarify the etiology of PH and right-sided heart failure (HF). It confirmed severe pulmonary hypertension (PH) with PAP 72/28 (mean 44) mmHg, severe TR, and dilated bilateral pulmonic trunk without the typical pattern of thromboembolism on pulmonary angiography; the pulmonary artery wedge pressure (PAWP) measured 4 (N 4-12) mmHg. Moreover, it showed all coronary arteries were patent, and LV EF was 80.88% without significant MR. Adding up, chest specialists felt that idiopathic pulmonary arterial hypertension (PAH) was the primary cause of right-sided HF. As recommended, the care team added sildenafil, a phosphodiesterase-5 (PDE5) inhibitor, to the bronchodilator therapy. The patient’s dyspnea improved in three days.
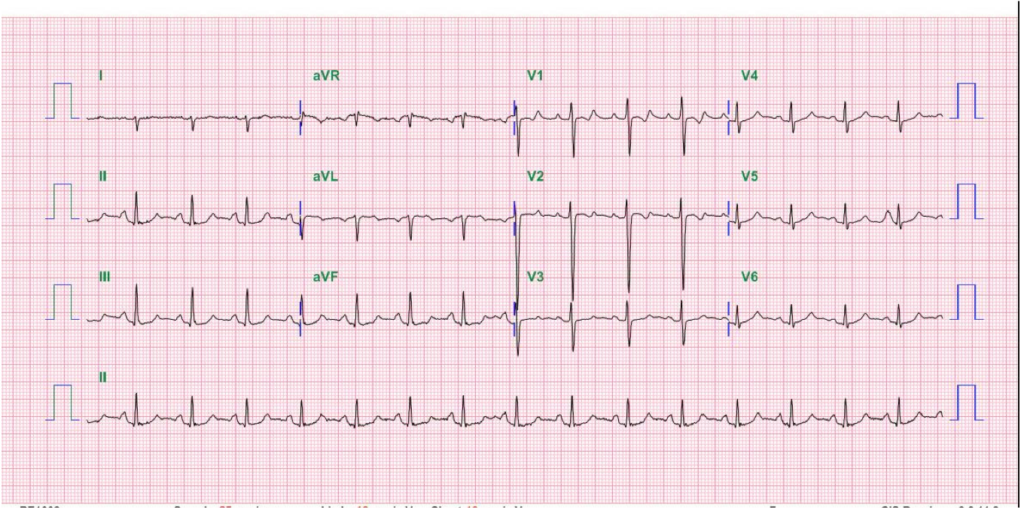
Diffuse low voltage
sinus rhythm at 94/min
Tall P in leads II (<2.5 mm), III, and aVF suggestive of
RA enlargement
Tall R wave in leads V1 and V2 with ST segment
flattening (strain pattern) in leads V1-V3 alongside
right axis deviation (+107º) reflecting RVH
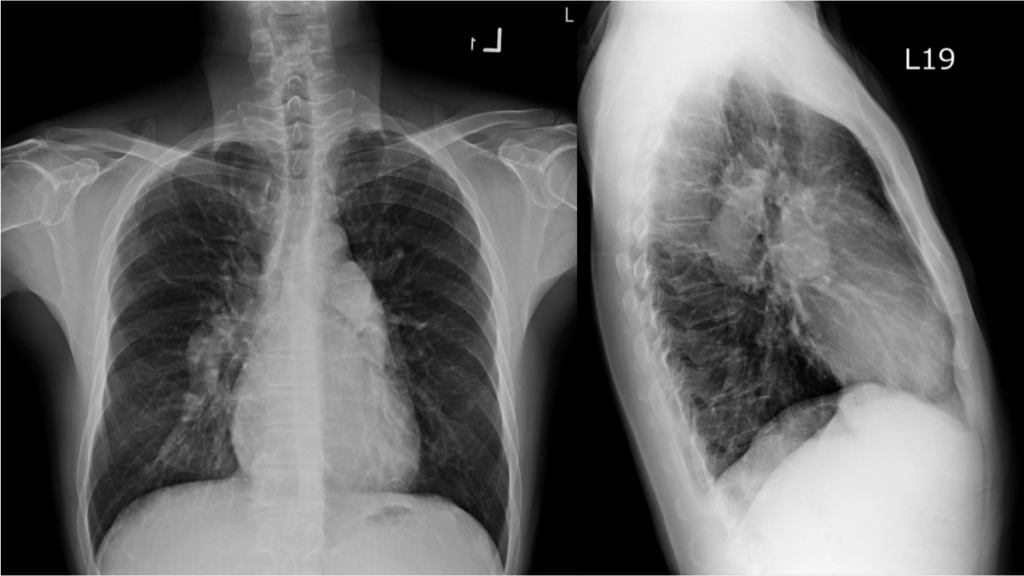
Hyperinflated lungs consistent with COPD
RA enlargement, RVH (obliteration of the retrosternal space on the lateral view)
Bilateral prominent pulmonary trunks, indicating severe pulmonary hypertension

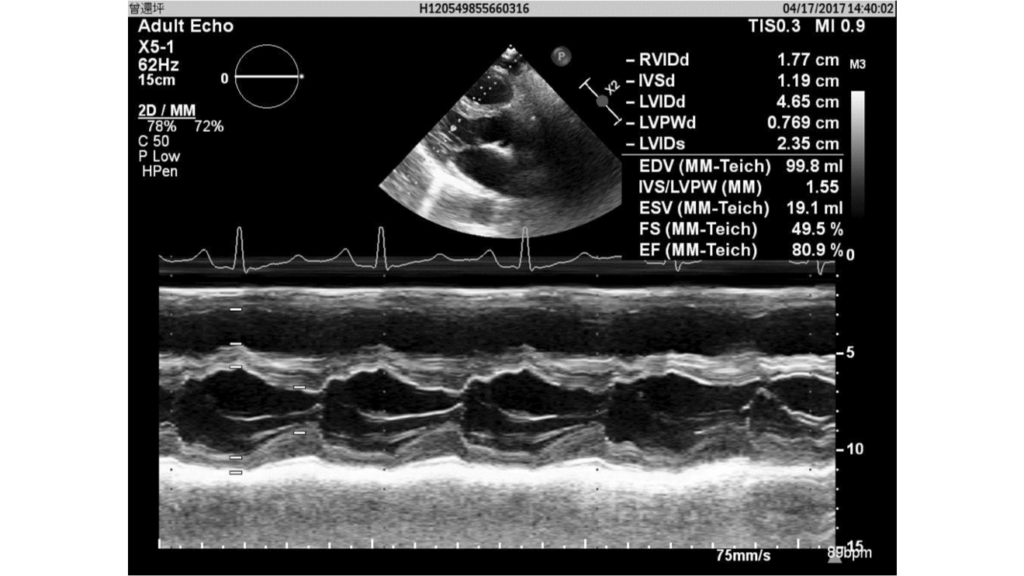



Dilated RA, RV, and LA (4.3 cm, LA/AO ratio 1.54)
Good LV contractility
Paradoxical septal wall motion
Asymmetric septal hypertrophy
TR, moderate
Severe pulmonary hypertension

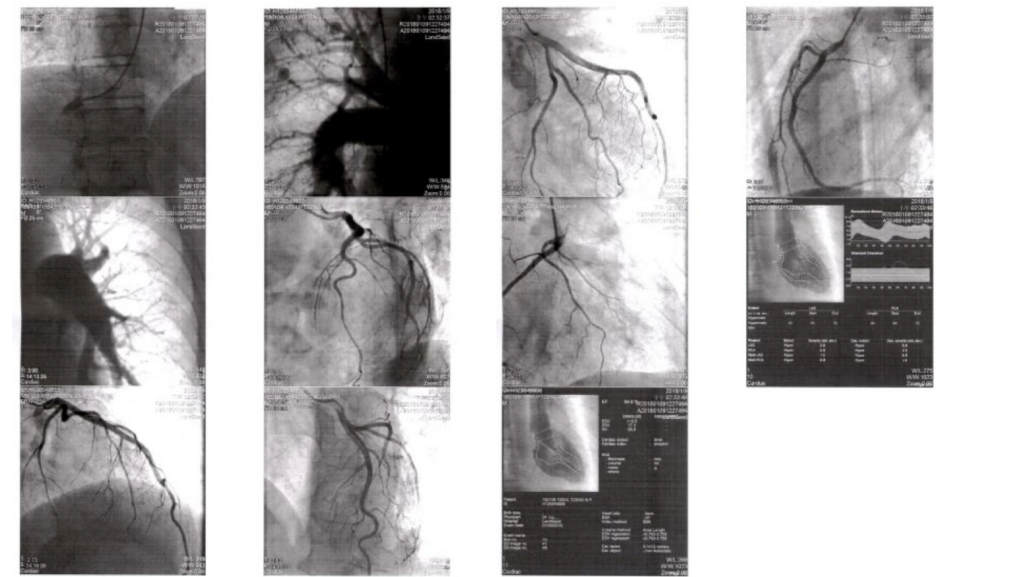
No significant O2 saturation step-up
(RA, RV and PA 65-66%; LA 100%, AO 96%)
Pulmonary artery wedge pressure (PAWP) 4 mmHg
Hepatic venous pressure gradient (HVPG) <10 mmHg***
Pulmonary hypertension PA 72/28 (mean 44) mmHg
Severe TR
Dilated bilateral pulmonic trunk
No typical pattern of thromboembolism
Patent coronary arteries
LV EF 80.88%
No MR
RA enlargement and RVH with strain shown on ECG align well with chest X-ray findings of PH associated with RA and RV enlargement and COPD. Diagnosing RVH on chest X-ray requires the lateral view to show obliteration of the retrosternal space, as illustrated in the present case. Echocardiography with Doppler remains the better means for identifying the structural and functional abnormalities of the heart’s four chambers. All the above findings call for further investigation into the etiology of right-sided HF with liver congestion on top of “alcoholic liver cirrhosis” (elevation of liver enzymes [SGOT 118 (N 5 to 40) U/L, SGPT 86 (N 7 to 56) U/L, Alk-P 164 (N 20-140) U/L, r-GT 162 (N 0-30) U/L with amonia 67.84 (N 26–94) mcg/dL, and previous negative workup for viral infection). COPD is likely the cause of diffuse low voltage.
Although an acute process cannot be excluded, severe PH of this extent (PA 72/28 [mean 44] mmHg) usually reflects its chronicity. The elevation of the D-dimer signals the possibility of acute pulmonary embolism, albeit only slightly elevated (655 [N <500] ng/ml)**. COPD appears mild, as the post-bronchodilator FEV1/FVC* remains within normal ranges (72 [70-80] %). Additionally, echocardiography suggests a possible presence of hypertrophic cardiomyopathy with LA enlargement. Accordingly, the care team needs to rule out other etiologies of PH responsible for right-sided HF, such as left-sided HF, chronic thromboembolic pulmonary hypertension (CTEPH), and primary pulmonary arterial hypertension (PAH), among others.
Specifically, the World Health Organization (WHO) has classified PH into five groups based on the cause, clinical presentation, hemodynamic characteristics, and treatment approach:
1. Group 1: PH due to PAH- idiopathic, heritable, drug and toxin-induced PAH, and PAH associated with conditions such as connective tissue diseases, HIV infection, portal hypertension, congenital heart diseases, schistosomiasis, and chronic hemolytic anemia.
2. Group 2: PH due to left-sided heart disease- PH due to systolic, diastolic, and valvular heart diseases.
3. Group 3: PH due to lung diseases and hypoxia- PH associated with COPD, interstitial lung disease, other pulmonary diseases with a mixed restrictive and obstructive pattern, sleep-disordered breathing, alveolar hypoventilation disorders, chronic exposure to high altitude, and developmental lung diseases.
4. Group 4: PH due to CTEPH and other pulmonary artery obstructions, including chronic thromboembolic obstruction and other conditions obstructing the pulmonary vasculature.
5. Group 5: PH with unclear and multifactorial mechanisms- various conditions such as hematological, systemic, and metabolic disorders.
All five groups share similar symptoms and signs related to the increased pressure in the pulmonary vasculature and RV strain. Still, the underlying causes, pathophysiology, clinical course, and management strategies significantly differ. Thus, the differential diagnosis and accurate classification of PH are crucial to guide appropriate therapy. In the present case, differentiating between PH secondary to COPD and PAH can be challenging. PH due to COPD is predominantly a consequence of hypoxic vasoconstriction, destruction of the pulmonary vascular bed due to emphysema, and antiproliferative response to inflammation. It is generally mild to moderate, with mean pulmonary arterial pressure rarely exceeding 35 mmHg. Typical findings include:
• Airflow obstruction in spirometry.
• Emphysematous changes in imaging.
• Possible hypoxemia and hypercapnia in arterial blood gas analysis.
On the other hand, PAH (Group 1 PH) has a complex pathophysiology involving endothelial dysfunction, smooth muscle proliferation, and thrombosis in situ, leading to obliteration of pulmonary arterioles. Thus, PAH has severe pre-capillary PH, typically with pulmonary arterial pressure much higher than that seen in COPD. Spirometry in PAH may appear normal or show a reduced diffusion capacity for carbon monoxide (DLCO). Imaging might not show any emphysematous changes. Echocardiography might reveal signs of RV strain or right-sided HF, but the timeline, progression, and severity often differ. Assessment of response to hypoxia and exercise, along with pulmonary function tests, imaging, and right heart catheterization, can provide valuable information to differentiate between the two forms of PH, as illustrated in the present case.
It should be noted that the presence of PH in a patient with COPD does not eliminate the possibility of concomitant PAH or vice versa, as these conditions can coexist, as illustrated in the present case. Therefore, comprehensive clinical evaluation and a multidimensional approach to diagnosis are crucial. Such diagnostic quandaries underscore the importance of referral to specialized centers for comprehensive assessment and treatment planning for patients with PH.
Despite the possible presence of hypertrophic cardiomyopathy, which might account for LA enlargement, there is no evidence of left-sided HF as PAWP measured only 4 (N 4-12) mmHg. The pulmonary specialists, therefore, recommend sildenafil for treating CTEPH in the present case. Sildenafil primarily acts by inhibiting phosphodiesterase-5 (PDE5), an enzyme found predominantly in the pulmonary vasculature as well as in the corpus cavernosum of the penis. PDE5 is responsible for the degradation of cyclic guanosine monophosphate (cGMP), a second messenger that plays a prominent role in mediating smooth muscle relaxation. By inhibiting PDE5, sildenafil precludes the degradation of cGMP, leading to augmented levels of this molecule. The increased cGMP levels result in further smooth muscle relaxation (vasodilatation) and enhanced blood flow to the pulmonary vasculature, thereby improving pulmonary circulation and reducing PA pressure. This latter property has led to the use of sildenafil in the management of PAH.
*The FEV1/FVC ratio: FEV1 stands for Forced Expiratory Volume in one second. It represents the volume of air a person can forcefully exhale in the first second of a forced breath. FVC, or Forced Vital Capacity, is the total volume of air that a person can exhale forcefully and rapidly after maximum inspiration. The FEV1/FVC ratio measures the speed at which lungs can be emptied and is used to assess bronchial obstruction. The FEV1/FVC ratio is usually about 0.7-0.8 or 70-80% in healthy adults. A lower-than-normal FEV1/FVC ratio can suggest an obstructive lung disease, such as COPD or asthma. A normal or high FEV1/FVC ratio, along with a reduced FVC, could mean a restrictive lung disease like pulmonary fibrosis. However, clinicians should be aware that various factors, especially age, can influence this ratio, as it typically declines with age.
**D-dimer levels can be elevated in a variety of clinical conditions. While it’s well known that high D-dimer levels can indicate thrombotic disorders, the test is nonspecific, and levels can be elevated in many different scenarios. Thus, while an elevated D-dimer level may require further investigation, it does not confirm the existence of any one particular disorder. 1. Thrombotic disorders- deep vein thrombosis (DVT), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC). 2. Cardiovascular conditions- myocardial infarction, stroke, and heart failure. 3. Infection and sepsis. Inflammatory states can activate coagulation cascades, leading to D-dimer elevation. 4. Malignancy. Certain cancers, especially metastatic ones, can cause a hypercoagulable state and elevate D-dimer levels. 5. Surgery and trauma. Surgical procedures and significant trauma, which involve tissue damage and subsequent activation of the coagulation system, can also cause D-dimer levels to rise. 6. Pregnancy. D-dimer levels can rise generally during pregnancy and be particularly elevated postpartum. 7. Age-related elevation. D-dimer levels can naturally increase with age due to a state of mild hypercoagulability seen in elderly patients. Misrepresentation of D-dimer results can occur if these conditions are not considered. Therefore, the clinical context is critical when interpreting D-dimer levels.
*** Hepatic Venous Pressure Gradient (HVPG) measurement is pivotal in assessing the grade of portal hypertension, which typically happens in chronic liver disease conditions. HVPG is calculated by subtracting the free hepatic venous pressure (FHVP) from the wedged hepatic venous pressure (WHVP) (HVPG = WHVP-FHVP). The median of three readings is taken as HVPG. FHVP is measured when the pressure-sensitive catheter is positioned at the distal portion of the hepatic vein without occluding flow. In contrast, WHVP is measured when the balloon catheter is inflated to occlude flow, thus providing a standing column to the portal vein via the hepatic sinusoid.4 In this regard, it is analogous to pulmonary wedged capillary pressure measurement. An HVPG score above the 5 mmHg indicates portal hypertension. Levels of >10 mmHg are indicative of clinically significant portal hypertension, while levels >12 mmHg are associated with a high risk of variceal bleeding. Direct HVPG measurements are invasive; they are primarily used in research settings or when non-invasive markers are not available or feasible. In the present case, the data was obtained during cardiac catheterization to clarify the etiology of PH.
Keywords:
phosphodiesterase-5 (PDE5) inhibitor, pulmonary hypertension, pulmonary arterial hypertension (PAH), right-sided heart failure.
UpToDate:
The epidemiology and pathogenesis of pulmonary arterial
hypertension (Group 1)
Treatment of pulmonary arterial hypertension (group 1) in adults:
Pulmonary hypertension-specific therapy
Treatment and prognosis of pulmonary arterial hypertension in
adults (group 1)

A 75-year-old man complained of worsening dyspnea with orthopnea for 2 hours. He had no chest pain or palpitations. He admitted dietary indiscretion and noted
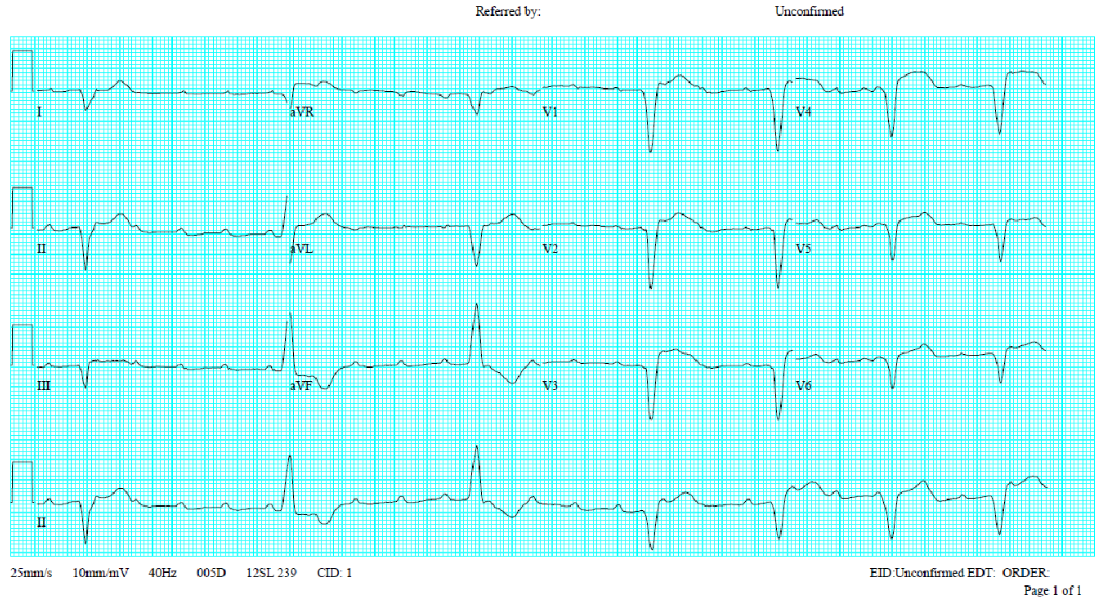
A 58-year-old man, who had been in good health, became infuriated after a quarrel with his wife for 20 minutes. He suddenly experienced severe chest
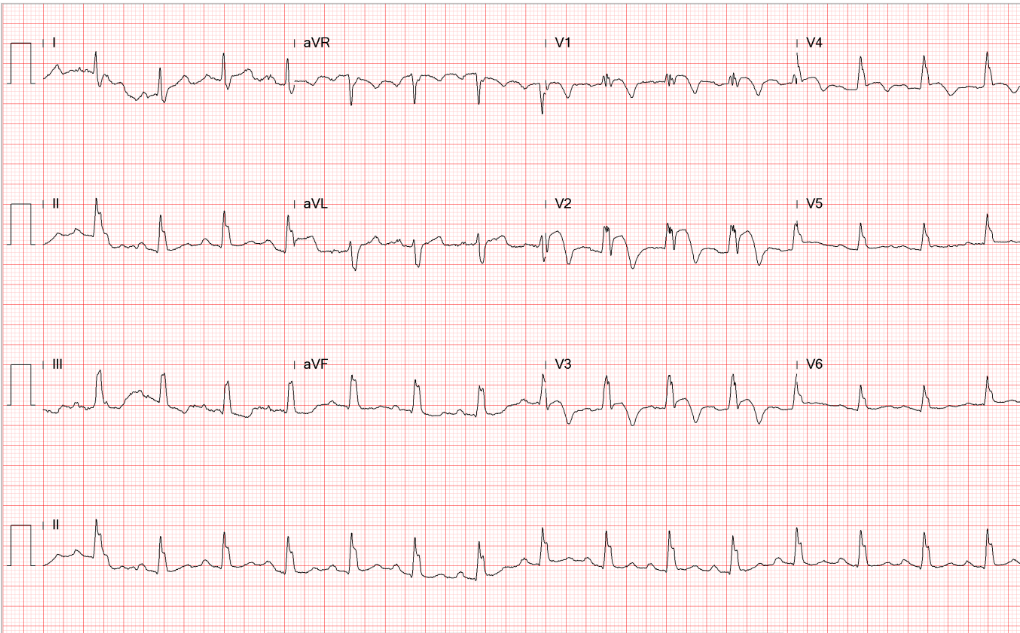
This 43-year-old woman was rushed to ER because of the sudden onset of substernal chest tightness accompanied by cold sweats, which lasted more than 30
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.