Case 33
This 35-year-old male was transferred from a local hospital for further evaluation after a biopsy of a hard, 4 cm in diameter lymph node in
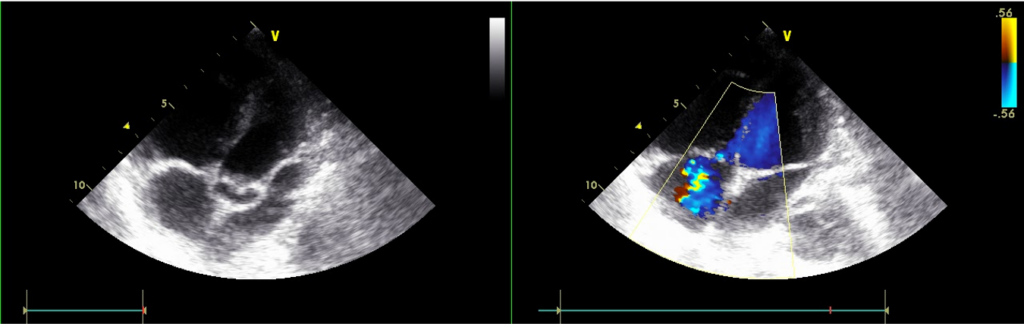
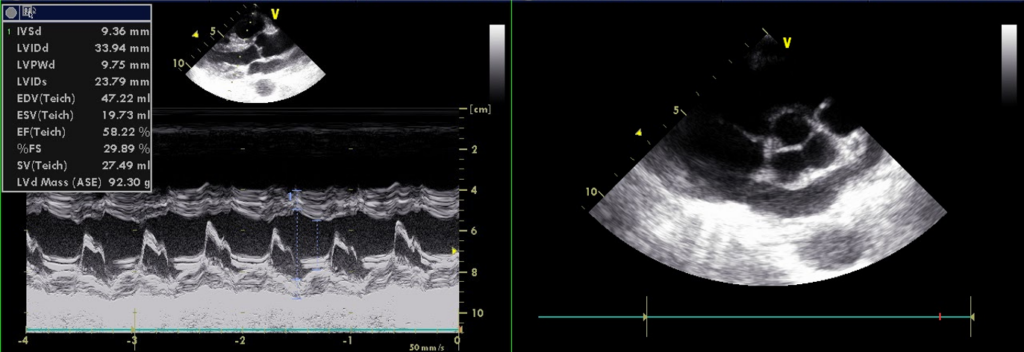
A 56-year-old man came to the Emergency Department because of worsening dyspnea with a productive cough for the past two weeks. Notably, he smoked one pack of cigarettes a day and had COPD with bronchiectasis for more than 20 years. He received treatment for pneumonia complicated by acute respiratory failure about six months prior and diuretic therapy for progressive edema involving the face, neck, and both legs one month earlier. He experienced no fever or chest pain. On arrival, he appeared cachectic* (BH 166.4cm, BW 43.1kg, BMI 15.57) in acute respiratory distress; BT/PR/RR measured 37 ºC/116/min/30/min with a SpO2 of 76 (N 95-100) %. BP was 103/71mmHg. JVD (11 cm H2O) was visible with a prominent CV wave suggestive tricuspid regurgitation (TR). PMI was not visible, but there was an RV lift with a Gr 3/6 holosystolic murmur best heard at the left lower sternal border. Characteristically, inspiration could increase the murmur intensity (a positive Carvallo’s sign indicative of TR). There were crackles and rhonchi at both lung fields. The liver margin was three finger-breaths palpable below the right costal margin and pulsatile. Clubbing fingers and bilateral 3+ leg edema were noted. ECG showed sinus tachycardia at 110/min and RA enlargement with RVH (P pulmonale). Chest X-ray revealed emphysema and diffuse fibrotic changes with reticular opacity with a honeycomb appearance at both lung fields compatible with bronchiectasis, pulmonary hypertension (PH), and possible secondary infection (pneumonia). ABG revealed respiratory acidosis with compensated metabolic alkalosis (pH of 7.39, pO2 67 (N 75-100) mmHg, pCO2 68.2 (N 32-45) mmHg, HCO3 35.7, BE 15.4 and O2 sat 92.5% (on nasal O2). Other pertinent laboratory data included Hb 13.9 g/dl, WBC 4.43 x103, Plt 265 x103, Na 136 mEq/L, K 4.3 mEq/L, BUN 16 mg/dl, Cr 0.95 mg/dl, SGOT 29 IU/L, SGPT 35 IU/L, troponin 0.01 ng/ml, CKMB 2.4 ng/ml. Subsequent echocardiography showed normal LA (17.55 mm) and LV chamber size with normal wall thickness, preserved LV systolic contractility without regional wall motion abnormality, RA and RV enlargement with an estimated PA systolic pressure of 67 mmHg, severe TR, and mild MR. As pneumonia was likely the cause triggering the worsening of respiratory failure associated with COPD, the care team quickly collected sputum for culture. Further, they empirically started antibiotic treatment with Curam (amoxicillin + clavulanic acid) while giving mucolytics, diuretics, and bronchodilators inhalation therapy to alleviate the breathing difficulty. The team realized the grim prognosis and continued supportive treatment.
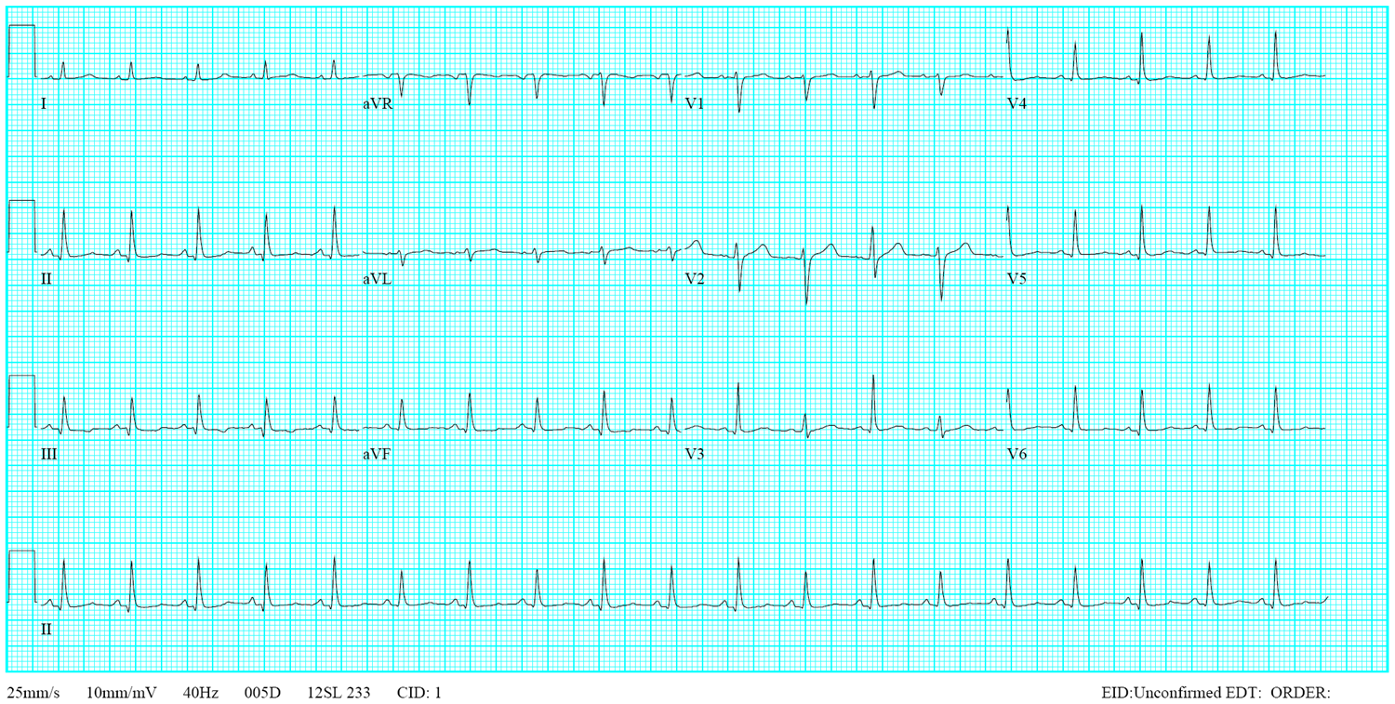
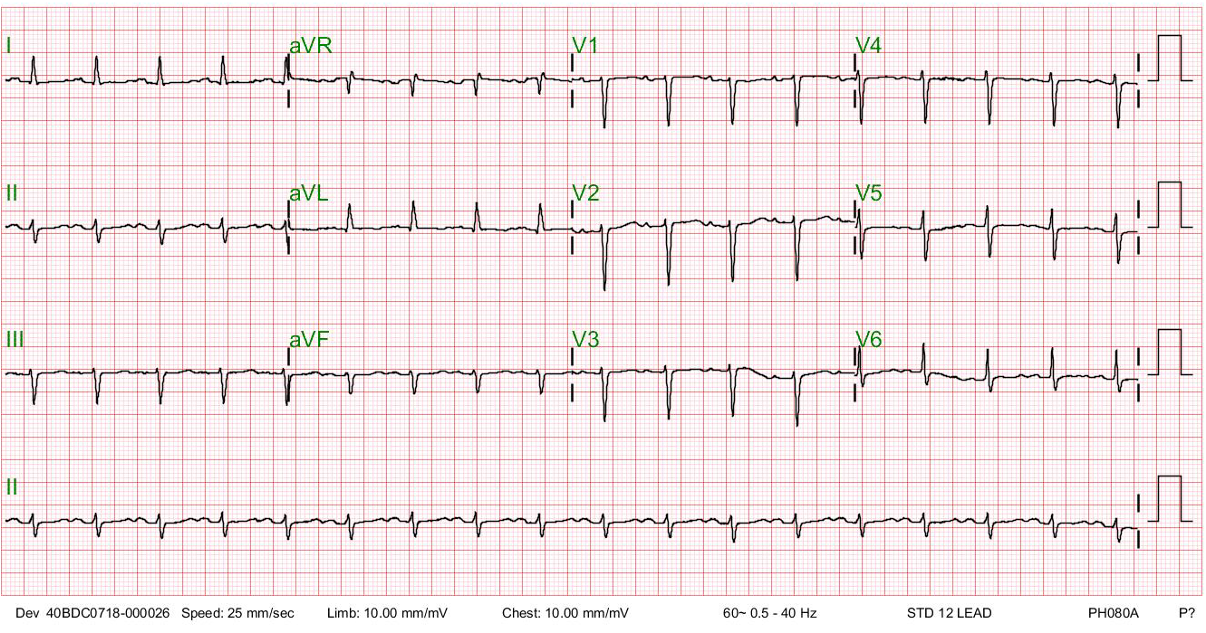
Sinus tachycardia at 110/min.
Tall peaked P waves in leads II, III, and aVF suggestive of RA enlargement
Deep negative P waves in the lead V1 r/o LA enlargement.
A qR pattern with right axis deviation (+121º) and slow R wave progression in V2-V6 (clockwise rotation) consistent with RVH and COPD
P pulmonale (RA enlargement with RVH and COPD)

Diffuse reticular opacity with a honeycomb appearance at both lung fields and fibrotic changes (more at the right upper lung compatible with bronchiectasis, r/o secondary infection (pneumonia)
Cardiomegaly
Prominent PA consistent with PH
Torturous aorta.
Blunting of costo-phrenic angles suggestive of small pleural effusion.
Slight scoliosis of the spine
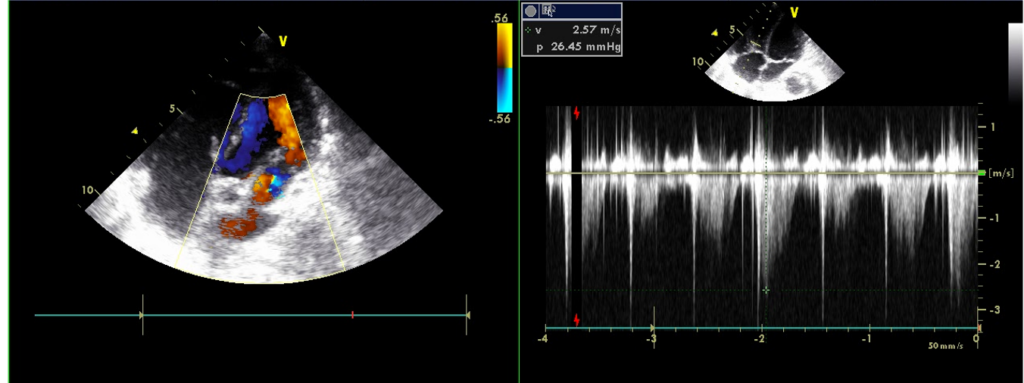
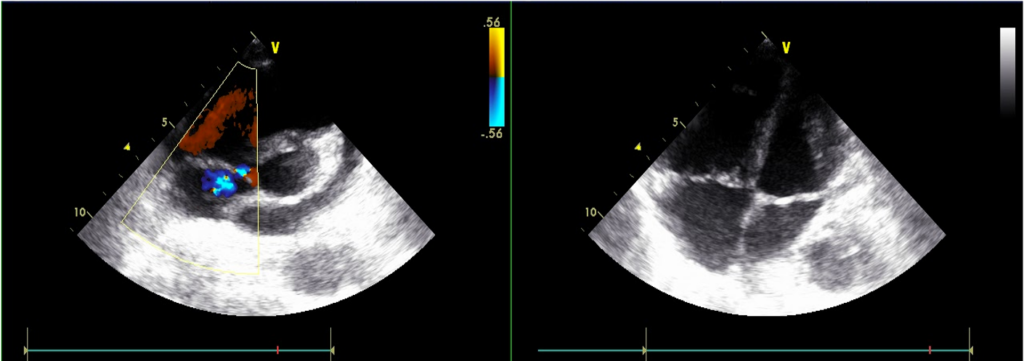
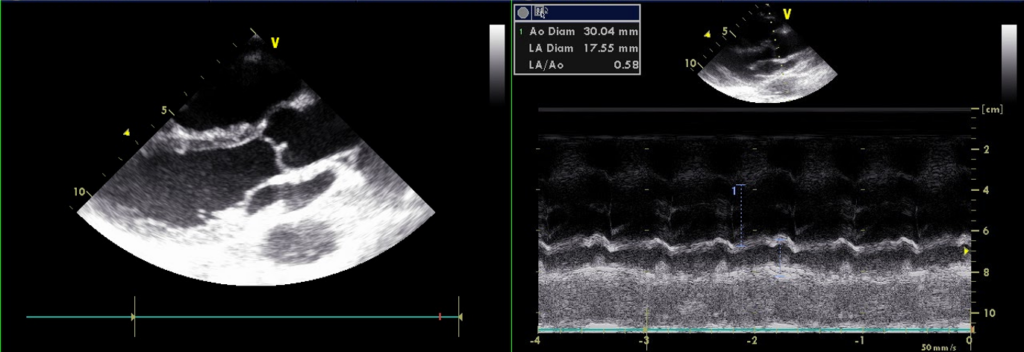
Normal LA (17.55 mm) & LV chamber size with normal wall thickness
No regional wall motion abnormality
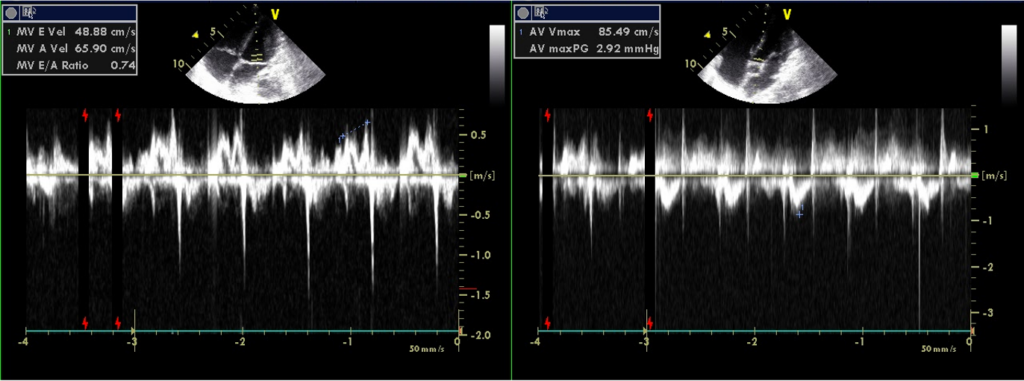
Preserved LV systolic contractility; E/A reverse, r/o LV diastolic dysfunction
RA and RV enlargement
Estimated PA systolic pressure 67 mmHg
Severe TR, mile MR
The peak P wave with an amplitude > 2.5 mm on the lead II indicates RA enlargement, and the qR pattern with right axis deviation (+121º) suggests RVH. The clockwise rotation of the heart (slow progression of the R wave from V2-V6) and relatively low voltage in V5 and V6 reflect the presence of COPD. COPD may obscure the dominant R wave in leads V1 to V3, usually seen in typical RVH. The RA enlargement associated with RH and chronic lung disease is called “P pulmonale.”
The finding of an entirely negative P wave in the lead V1 is intriguing. The sinoatrial node is located at the junction of RA and the superior vena cava. Anatomically, the LA is positioned posteriorly and slightly inferiorly to the RA, activated through the Bachmann’s bundle (the interatrial band) from the RA. Thus, in leads VI and V2, the P wave (atrial activation) usually consists of two parts: the initial upright part (reflecting RA activation directed anteriorly) is followed by an inverted or negative portion of the P wave (reflecting LA activation directed posteriorly). However, a narrow but prominent, entirely negative P wave in the lead V1 may paradoxically represent RA rather than LA enlargement.** This occurs mainly when the RA is located below the level of the V1 electrode, as illustrated in the present case with COPD (see chest X-ray). Echocardiography confirms RA but no LA enlargement (LA 17.55 mm in diameter).
This patient has a “cor (heart) pulmonale” medical condition. It refers to PH-induced altered RV structure (e.g., hypertrophy or dilatation) and impaired RV function associated with chronic lung disease and hypoxemia, as shown in the present case with RV failure associated with COPD and bronchiectasis. Specifically, long-term effects of hypoxic pulmonary vasoconstriction upon RV can cause PH and subsequent RA and RVH. Therefore, the ECG pattern associated with “cor pulmonale” consists of “P pulmonale “ (i.e., the peak P wave amplitude > 2.5 mm in leads II, III, and aVF or > 1.5 mm in V1 and V2), RVH (e.g., right axis deviation, qR wave in lead V1, dominant R wave in leads V1 to V3). However, if it is associated with hyperexpanded emphysematous lungs (i.e., COPD), clockwise rotation and low voltage QRS complexes may be seen, especially in the left precordial leads (V4-6). As illustrated in the present case, the clockwise rotation due to COPD may also obscure or mask the dominant R wave in V1 to V3. While some experts argue about the inclusion of RV dysfunction due to pulmonary vascular disease (i.e., Group 1 PH: primary pulmonary arterial hypertension [PAH])**, most agree that “cor pulmonale” should not include RV dysfunction or right-sided HF resulting from left-sided HF or congenital heart disease (e.g., pulmonary stenosis, Tetralogy of Fallot). Clinically, patients with “cor pulmonale” can manifest SOB, fatigue, chest pain, fainting, and even sudden death.
Bronchiectasis is a medical condition when the bronchioles of the lungs become damaged with gradual thickening of the inner wall due to repeated infection or a medical condition related to the lungs. Consequently, the build-up of tenacious mucus and bacteria causes blockage of airways, resulting in a vicious cycle of infection and bronchial damage, causing breathing difficulty. The condition, if unrecognized or not adequately treated, will lead to recurrent pneumonia, emphysema, empyema (accumulation of pus in the pleural space), lung abscesses, respiratory failure, and cor pulmonale. Besides shortness of breath, clinically, patients with bronchiectasis often manifest chronic productive cough with a large amount of thick mucus with or without blood, thickening of the skin under fingers and toes (clubbing due to chronic hypoxia), weight loss (cachexia)*, and respiratory failure like in the present case.
*Cachexia is characterized by unintentional weight loss, muscle wasting, and general physical weakness. Chronic inflammation, hormonal imbalances, and metabolic abnormalities can cause this condition. It is usually associated with chronic diseases such as cancer, HF (as in the present case), and HIV/AIDS infection.
**The ECG criteria for LA enlargement (P mitrale):
1. Bifid (bimodal) P wave with > 40 ms between the two peaks in lead II (P
mitrale)
2. Total P wave duration > 110 ms in lead II.
3. Biphasic P wave with terminal negative portion > 40 ms duration in lead
V1
The term “mitrale” means bishop’s hat, similar to the shape of the P wave on the ECG. Intraatrial or interatrial delay or block refers to conduction delay between RA and LA, usually at the level of the Bachmann bundle. Differential diagnosis between intraatrial or interatrial conduction disturbance versus LA enlargement based on the P wave morphology is difficult. Under the circumstances, echocardiography is to provide an accurate assessment of the LA size, as illustrated in the present case.
***WHO classification of pulmonary hypertension (PH)
Group I- Pulmonary arterial hypertension (PAH)
Group II- PH due to left-sided heart diseases
Group III- PH due to lung diseases and hypoxia
Group IV- PH due to chronic thromboembolic disease
Group V- PH with unclear and multifactorial mechanisms
Group III-PH can be due to:
1. Obstructive lung disease (e.g., chronic obstructive pulmonary disease or
bronchiectasis)
2. Restrictive lung disease (e.g., interstitial lung disease, kyphoscoliosis)
3. Other lung diseases with mixed obstruction and restriction (e.g.,
pulmonary fibrosis with emphysema)
4. Hypoxia without lung disease (e.g., high altitude, sleep-disordered
breathing, obesity hypoventilation)
5. Developmental lung disorders (e.g., bronchopulmonary dysplasia,
congenital lobar emphysema)
Keywords:
bronchiectasis, chronic obstructive pulmonary disease (COPD), cor
pulmonale, pulmonary hypertension
UpToDate:
Clinical manifestations and diagnosis of bronchiectasis in adults
Bronchiectasis in adults: Treatment of acute and recurrent
exacerbations
Pulmonary hypertension due to lung disease and/or hypoxemia
(group 3 pulmonary hypertension): Treatment and prognosis

This 35-year-old male was transferred from a local hospital for further evaluation after a biopsy of a hard, 4 cm in diameter lymph node in

A 57-year-old woman with a long-standing history of bronchial asthma and COPD was admitted because of intermittent episodes of dyspnea associated with palpitations and general
A 41-year-old Taiwanese woman, G3P1, developed progressive dyspnea on exertion, PND, and orthopnea for five days after she gave birth to a healthy baby boy
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.