Case 20
An 81-year-old man came to the Emergency Department (ED) after one day of having progressive dyspnea with orthopnea and had diffuse itchy skin for more than a week. He had chronic heart failure (HF) with atrial fibrillation (AF) due to dilated cardiomyopathy for over 15 years. Reportedly, two years ago, he had a stroke, and five months prior, he underwent video-assisted thoracoscopic pleurectomy and decortication with pericardiectomy for presumed tuberculosis (TB) infection. He was on valsartan and furosemide for HF, dabigatran, and pitavastatin for s/p stroke. He had taken pyrazinamide, rifampicin, plus isoniazid for anti-TB therapy for five months, which might have caused the generalized skin rash he had recently developed. At ED, he was in moderate respiratory distress. BT/PR/RR was 35.6° C, 163/min, irregular, and 20/min, respectively, and BP measured 109/56 mmHg. Physical findings were compatible with biventricular failure, i.e., JVD 12 cm, displaced PMI to LAAL, a Gr 3/6 pan systolic murmur at the apex, liver span 13 cm with positive hepatojugular reflux, bilateral crackles in both lungs and 3+ leg edema. ECG showed diffuse low voltage in AF with a ventricular rate of 177/min. There was a clockwise rotation of the heart alongside diffuse low voltage, suggesting chronic obstructive lung disease (COPD). Chest X-ray revealed cardiomegaly, RA, LA, and LV enlargement with prominent PA (bilateral) indicative of pulmonary hypertension; there was also pulmonary congestion with pleural effusion (L>R). Overall, the findings could not exclude pericardial effusion. Pertinent laboratory data included ABG: pH 7.486, pCO2 19 mmHg, pO2 102 mmHg, HCO3 14 mmol/L, B.E. -5.9 mmol/L, SaO2 98.5 %; Hb 12.1 gm%, WBC 5800, Plt 110 K, AST 32 IU/L, ALT 19 IU/L, BUN 36 mg/dl, Cr 1.4 mg/dL, Na 140 mEq/L and K 2.9 mEq/L, PT 14.0 (N 10-13) sec, APTT 36.9 (N 25-35) sec, INR 1.33 (<1.1), troponin-I <0.05 ng/mL, BNP 1560 (N 150) pg/mL. Echocardiography confirmed LA, RA, and LV enlargement with impaired global contractility of LV (EF: 40.1 %). Notably, there was associated regional wall motion abnormality suggestive of atherosclerotic coronary heart disease (CHD). PI was moderate, MR moderate to severe, and TR was severe; PA measured 63 mmHg. Fortunately, pericardial effusion was minimal, and there was no sign of RV diastolic collapse*. The care team used intravenous verapamil to slow the ventricular rate of AF while orally adding digoxin and corrected hypokalemia with K supplement and later with oral spironolactone. The patient gradually improved and could return home in two weeks with a new medical regimen.
ECG
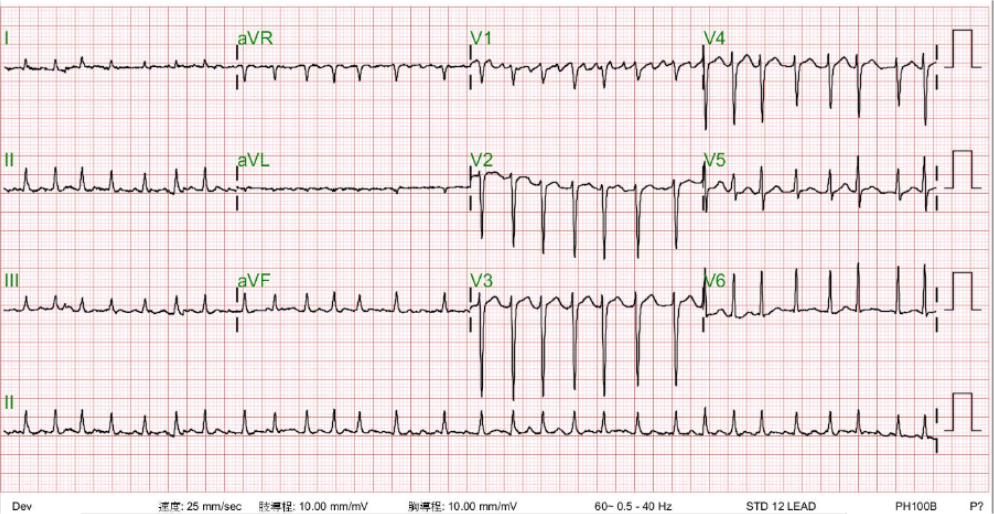
Atrial fibrillation with a ventricular response of 177/min
Normal axis (+60°)
Diffuse low voltage with slow progression of R waves in leads V1-V4 suggestive of the presence of COPD.
Cyclical variation of the QRS amplitude (no QRS alternans) most prominent in lead V4, likely caused by a respiratory effect with increased lung volume (e.g., COPD).
LVH likely
Nonspecific ST-T wave changes
Chest X-ray
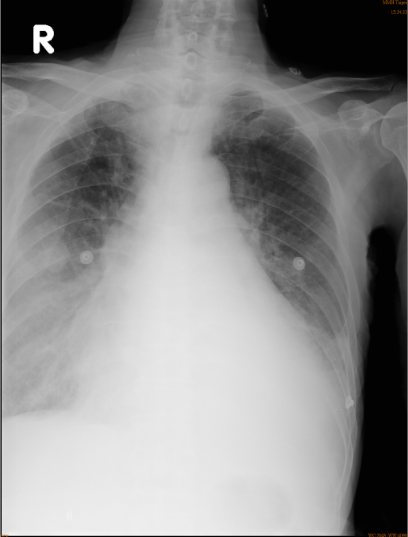
Cardiomegaly with Prominent pulmonary arteries (PA) (bilateral) suggestive of pulmonary hypertension.
Trachea deviation, RA, LA, and LV enlargement (r/o pericardial effusion)
Opacification of lower lung fields suggests bilateral pleural effusion (L>R).
A round soft tissue nodule (2 cm in diameter) in the right middle lung field (r/o lung tumor).
Chest X-ray 3 days after treatment
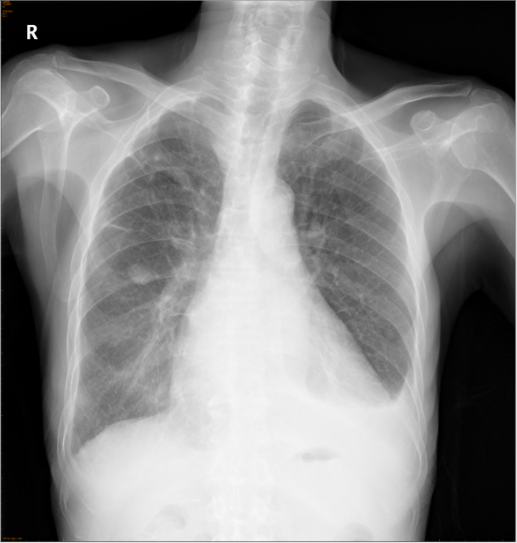
A marked improvement as compared to 3 days ago.
Echocardiography
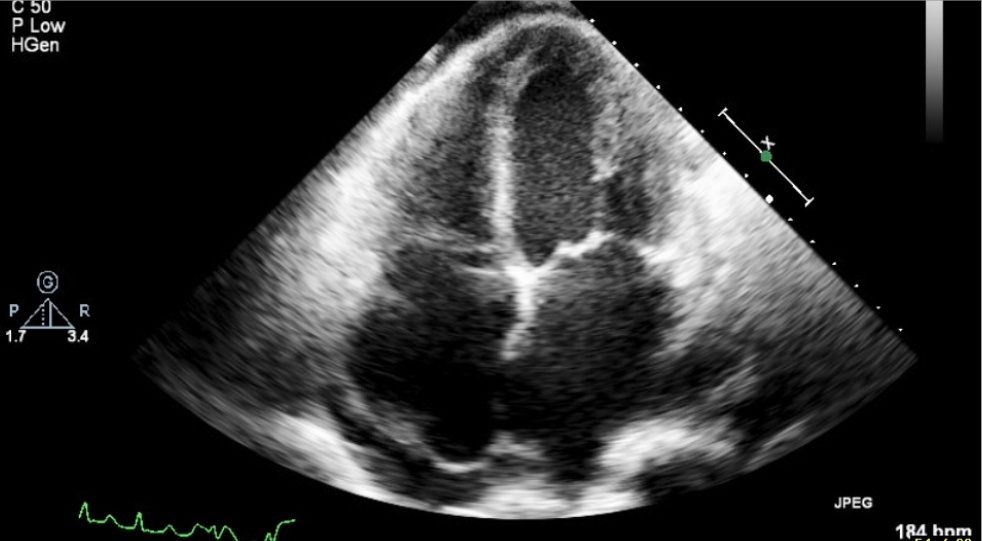
LA, RA, and LV enlargement.
Impaired global contractility of LV with EF: 40.1 %.
Regional wall motion abnormality, suspect coronary artery disease.
Impaired LV diastolic function
Calcification of aortic valve with moderate aortic regurgitation (AR)
Moderate-to-severe mitral regurgitation (MR).
Moderate pulmonary insufficiency (PI) (estimated PA systolic pressure 63 mmHg)
Severe tricuspid regurgitation (TR).
Moderate pulmonary hypertension (PH).
Minimal amount of pericardial effusion without right ventricular diastolic collapse*.
Myocardial perfusion scan with SPEC study
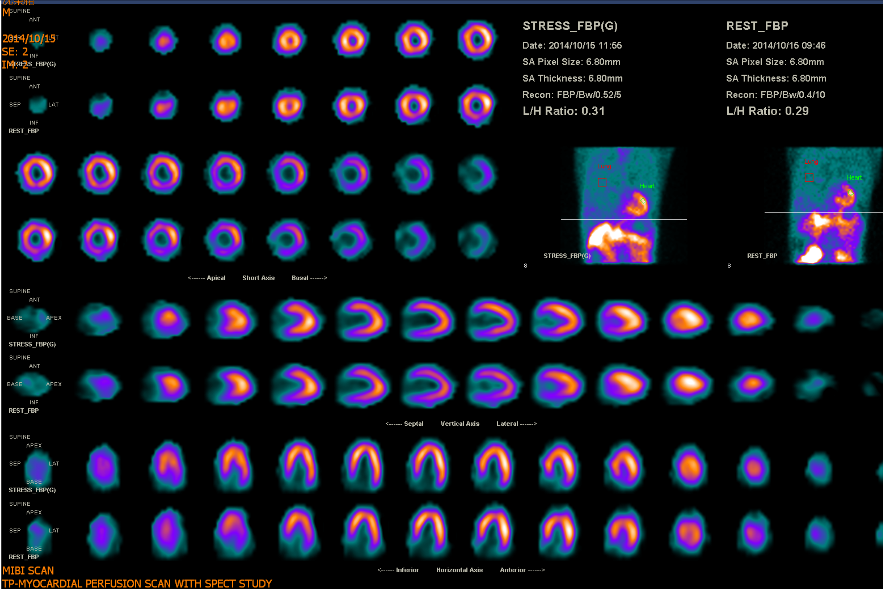
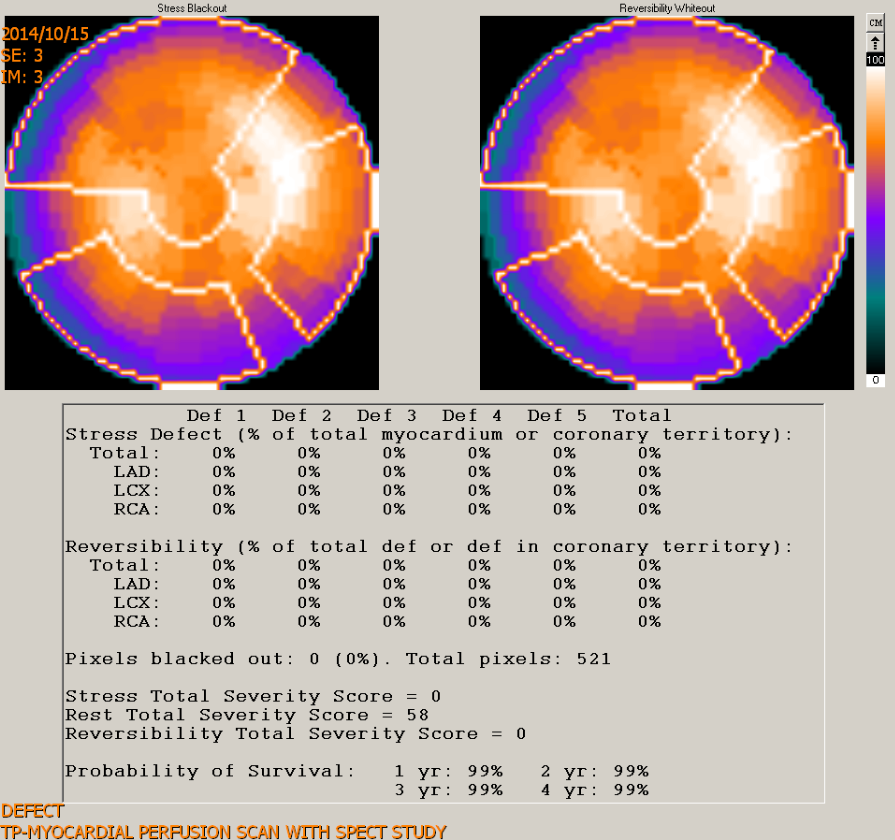
Clinical interpretation
Careful reading of the ECG recognizes AF with a ventricular rate of 177/min. Cyclical variation of the QRS amplitude (most prominent in lead V4) syncs with respiratory movements. As the chest X-ray shows, this phenomenon is likely caused by an increased lung volume (e.g., COPD). There are no QRS alternans. Although nonspecific, QRS alternans can be associated with pericardial effusion, cardiac tamponade, dilated cardiomyopathy, supraventricular tachycardia, electrolyte imbalance, etc. In the present case, echocardiography reveals no significant pericardial effusion or signs of cardiac tamponade.
The rapid ventricular rate of AF may be the cause of or triggered by worsening HF. Since its presence is chronic, the critical issue is not cardioversion but rather which drug would be safe for slowing the rapid ventricular response in the clinical context of biventricular failure with LVEF of 40.1 % and COPD. A beta-blocker or a calcium-channel blocker can be used in this situation, but the dosing and rate of administration should be closely monitored. Under the circumstances, the care team decided to digitalize the patient and use intravenous verapamil to slow the ventricular rate of AF.
With right-sided HF, as evidenced by JVD and palpable liver and leg edema, liver enzymes are normal, but PT, INR, and APTT (PT 14.0 sec, INR 1.33, APTT 36.9 sec) are slightly elevated. Although liver injury due to liver congestion may induce abnormal coagulation parameters, dabigatran, a direct oral anticoagulant that inhibits thrombin, may be the culprit, especially for APTT. However, these parameters are not reliable indicators of the anticoagulant effect of dabigatran. Specific laboratory tests can assess dabigatran’s anticoagulation impact, like dilute thrombin time (dTT) and ecarin clotting time (ECT). When using dabigatran, it is essential to evaluate renal function (kidneys eliminate 80-85% of dabigatran) and to watch for signs of bleeding or thromboembolic events. Regular follow-up and adherence to dosing guidelines are necessary to ensure dabigatran’s safe and effective use, such as with BUN 36 mg/dl and Cr 1.4 mg/dL, as seen in the present case.
HF can be regarded as a multifactorial clinical syndrome affecting and involving multiple organs, including the brain, lungs, liver, kidney, gastrointestinal tract, and even the skeletomuscular and immune systems. ABG showing respiratory alkalosis with compensatory metabolic acidosis aligns with the initial stage of HF with hyperventilation (RR 22/min). The heart and lungs are anatomically close to each other for transporting and exchanging unoxygenated and oxygenated blood. Besides the hemodynamic effect, there are short-term and long-term neurohormonal interactions (cross talk). Reportedly, CHD is prevalent in patients with COPD because of hypoxia-induced inflammatory reactions, accelerating the process of atherosclerosis in the vascular endothelium. Although echocardiography revealed segmental hypokinesis in the present case, a myocardial perfusion scan with SPEC study was negative for significant myocardial ischemia.
Similarly, renal dysfunction (BUN 36 mg/dl and Cr 1.4 mg/dL) is likely a reciprocal response of the kidneys to renal hypoperfusion resulting from HF-induced reduction in cardiac output, the so-called cardiorenal syndrome. Cardiac output represents the primary regulator of renal function, as measured by the estimated glomerular filtration rate (eGFR). More specifically, HF affects renal function via the activation of the neurohormonal renin-angiotensin-aldosterone system (RAAS), aiming at maintaining adequate organ perfusion in the whole body, including the kidney. The activated RAAS can exert long-term effects on cardiac and vasculature remodeling, leading to chronic HF. The heart, in turn, releases NT-proBNP and BNP through its endocrine function to counteract adverse effects produced by RAAS. Similar crosstalk occurs between the heart and other organ systems (see Reference).
Among the causes of worsening HF, dietary indiscretion is the most seen. Other causes include myocardial ischemia or MI, cardiac arrhythmias (tachycardia or bradycardia), medical nonadherence, infection, anemia, uncontrolled hypertension, adverse effects of medications (e.g., beta-blockers, calcium-channel blockers, antiarrhythmic drugs, NSAIDs, anti-TNF antibodies), worsening renal dysfunction, etc. AF with a rapid ventricular rate can be the cause or a result of worsening HF in the present case. However, the addition of new medications, especially those with depressive effects on the myocardium, should be identified. The patient had been taking pyrazinamide, rifampicin, and isoniazid medications for anti-TB therapy for five months. While these medications are not known to have direct negative inotropic effects on the heart, they can potentially cause adverse effects on the cardiovascular system, including developing or exacerbating HF. Close monitoring is necessary during TB treatment to ensure patient safety.
*Diastolic collapse of the RV can be seen in conditions such as cardiac tamponade, which is the accumulation of fluid or blood in the pericardial sac that compresses the heart chambers. The increased pressure within the pericardial sac can cause the RV to collapse during diastole. Other potential causes of the diastolic collapse of the RV include constrictive pericarditis, which is the thickening and stiffening of the pericardium, and severe RV hypertrophy, which can occur in conditions such as arrhythmogenic right ventricular cardiomyopathy. The presence of diastolic collapse of the RV on echocardiography is an important finding that suggests significant pathology and requires further evaluation to determine the underlying cause. Additional diagnostic tests, such as cardiac catheterization or magnetic resonance imaging (MRI), may be necessary to confirm the diagnosis and guide appropriate management. It is important to note that the interpretation of echocardiographic findings, including the diastolic collapse of the RV, should be done in the context of the patient’s clinical presentation, medical history, and other imaging or diagnostic findings to arrive at an accurate diagnosis and guide appropriate treatment.
Keywords:
cardiorenal syndrome, dabigatran, renin-angiotensin-aldosterone system
References
UpToDate:
Management of the patient with COPD and cardiovascular disease
Cardiorenal syndrome: Definition, prevalence, diagnosis, and pathophysiology
Ciccarelli M et al. Reciprocal organ interactions during heart failure: a position paper from the ESC Working Group on Myocardial Function. Cardiovascular Research, 2021;117:2416–2433.
Leave a comment
Case 2
A 58-year-old man, who had been in good health, became infuriated after a quarrel with his wife for 20 minutes. He suddenly experienced severe chest
Case 18
This 69-year-old man had been in good health until two days before admission when he returned from a beach. He experienced general weakness with chest
Contact
If you have further questions or have interesting ECGs that you would like to share with us, please email me.