Case 28
This 92-year-old man complained of progressive dyspnea with PND, productive cough with whitish sputum, and bilateral leg edema for the past few weeks. He was a poor historian. Except for COPD, he recalled diabetes mellitus (DM) disclosed by a local physician two weeks prior. At the Emergency Room, he appeared apprehensive and was in moderate respiratory distress. BT/PR/RR were 36.5℃, 102/min, and 20/min, respectively, and blood pressure measured 117/68 mmHg. The chest A-P diameter was increased, and the rest of the physical findings were consistent with biventricular heart failure (HF). These included JVD 10 cm H2O, displaced PMI at 6th intercostal space 2cm left to the left midclavicular line, Gr 2/6 systolic murmur at the left sternal border, and an S3 gallop, scattered rhonchi throughout, bilateral rales at lower one-third bases, liver margin palpable two finger breath below the right costal margin, and 2+edema at both lower legs. ECG showed low voltage in limb leads, sinus rhythm at 95/min, and LVH; chest X-ray revealed RA, LA, and LVH with pulmonary congestion and bilateral pleural effusion. Pertinent laboratory data showed Hb 12.3 g/dl, Hct 32%, WBC 5600, Na 129 mEq/L, K 4.4 mEq/L, Cl 93 mEq/L, BUN 48 mg/dl, Cr 1.4 mg/dl, estimated GFR 47.4 ml/min, TBil 1.7 mg/dl with dir 0.6 mg/dl, ALKP 215 (N 38-126) IU/L, Albumin 3.3 (3.5-5.0) g/dl, SGOT 72 IU/L, SGPT 112 IU/L, r-GT 356 IU/L, troponin-I 0.09, CKMB 8.3 (N <5.4) ng/mL, Glucose (AC) 439 mg/dl, HbA1c 11.1%, PT 12.4 (N 8.0-12.0) sec, BNP 2320 pg/ml, CRP 2.5 (N <0.8) mg/dl, TSH 3.69 (N 0.25-4.00) μIU/ml, Free T4 1.43 (N 0.89-1.79) ng/dL, Anti HBs (+) / HBsAg (-), HCV Ab (-), cold hemagglutinin 16x+ (N < 32x+).* A pleural tap showed the effusion to be transudate** (appearance transparent, specific gravity 1.010, protein 14.6 g/L, glucose 426 mg/dl, LDH 58 U/L). Echocardiography with Doppler revealed critical aortic stenosis (AS) (aortic valvular area [AVA] measured 0.40~0.52 cm2) with RA, LA, and LV enlargement, an estimated LVEF of 48.4 %, moderate to severe MR, and pulmonary hypertension (systolic PA pressure 54 mmHg). The care team started diuretics with IV furosemide and oral spironolactone and added metformin and DPP-4 therapy to control biventricular HF and hyperglycemia, respectively. The patient responded well clinically (Chest X-ray 2) within one week. BNP dropped to 1260 pg/ml, and except for slight hypokalemia, kidney and live function test results had also significantly improved (Na 136 mEq/L, K 3.3 mEq/L, Cl 93 mEq/L, BUN 34 mg/dl, Cr. 1.1 mg/dl, estimated GFR 62.6 ml/min, TBil 1.5 mg/dl with dir 0.5mg/dl, SGOT 47 IU/L, SGPT 80 IU/L, r-GT 159 IU/L). Urinalysis findings, except for glucose 3+, were unremarkable. Reckoning the risk of aortic valve replacement based on his age and comorbidities, the care team entertained transcatheter aortic valve replacement (TAVR) as the minimum invasive procedure.*** However, the patient declined and signed for DNR (do not resuscitate) after deliberation with his family.
ECG
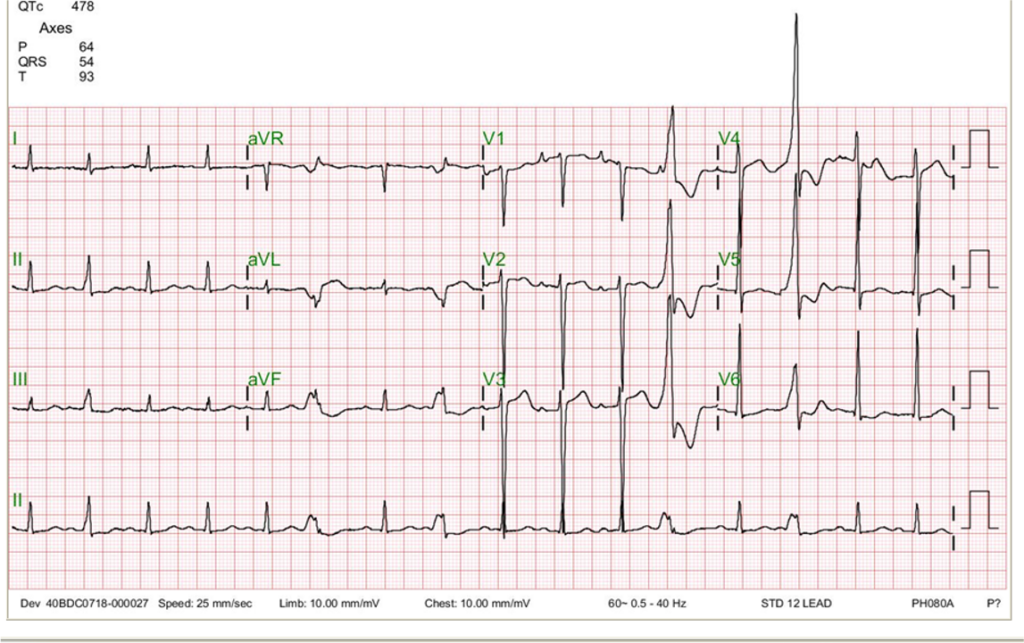
Low voltage in limb leads
Sinus rhythm at 95/min
LVH
premature ventricular beats originating at the LV outflow tract
Chest X-ray
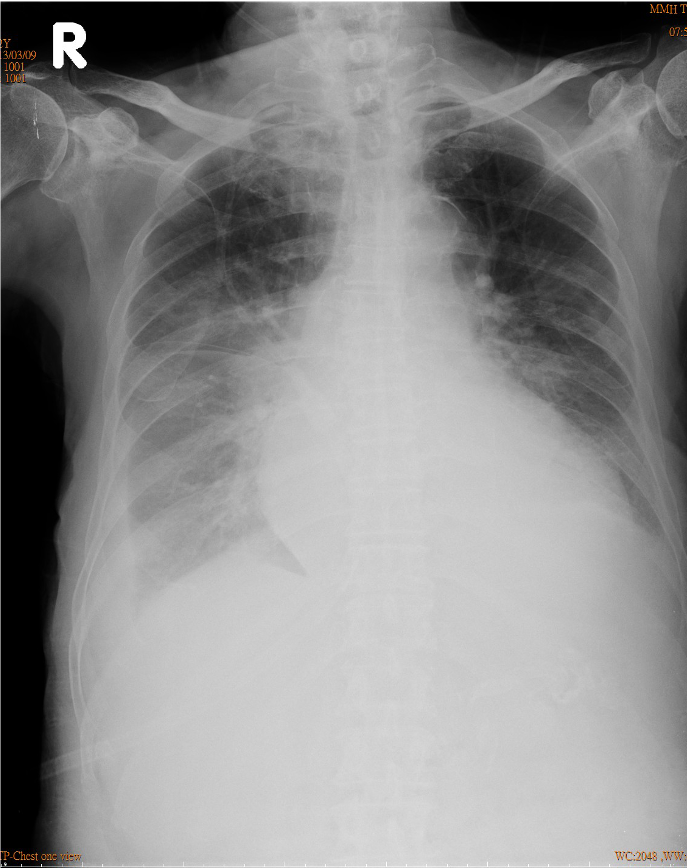
Increase in cardiothoracic ratio suggestive of cardiomegaly, r/o pericardial effusion
Calcified aortic knob.
Prominent PA indicative of pulmonary hypertension.
RA and LA enlargement.
Pulmonary congestion with bilateral pleural
effusion (R>L).
Chest X-ray 2

Compared to the previous one taken on admission, there is much improvement in pulmonary congestion and pleural effusion.
Echocardiography
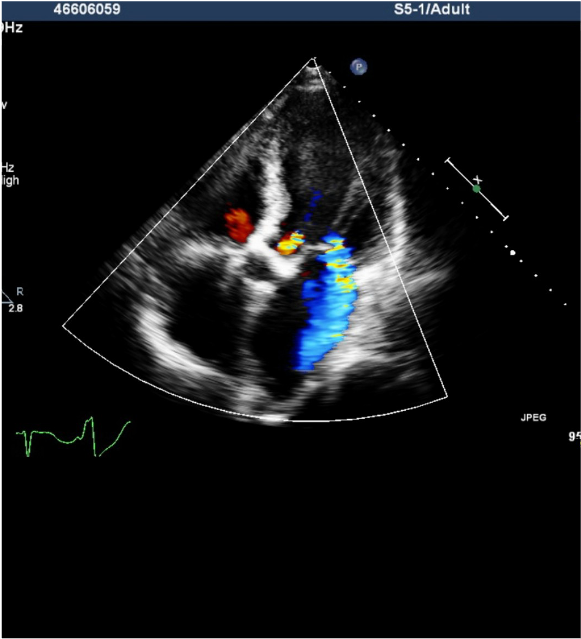
RA, LA, and LV enlargement.
Impaired systolic and diastolic function of LV with an EF of 48.4 %.
Regional wall motion abnormality, suspect coronary artery disease.
Moderate to severe MR, mild AR, and moderate TR.
Pulmonary hypertension with systolic PA pressure of 54 mmHg
Severe AS with calcification with a peak systolic gradient of 60 mmHg.
Estimated AVA 0.40~0.52 cm2.
Clinical interpretation
The uncovered calcific AS can account for the LVH pattern with strain (secondary ST-T wave changes) seen on the ECG. On the other hand, COPD is likely the cause of the low voltage of the QRS complex in limb leads. The development of LVH is a longstanding process, which, if unrecognized and inadequately managed, as in the present case, can lead to HF, ventricular arrhythmias, and even sudden cardiac death. COPD and pleural effusion often interfere with assessing the cardiac status noted on the chest X-ray. Under the circumstances, echocardiography helps rule out significant pericardial effusion. It notably identifies severe AS due to the calcific aortic valve and its related hemodynamics (LVEF, chamber enlargement, etc).
The causes of AS in adults can be attributed to three primary etiologies: First, calcium deposit build-up on the aortic valve leading to calcific AS is the most common, particularly in those over 65 years (an aging process); Second, degenerative changes of congenital bicuspid aortic valve eventually induces AS. Third, aortic valvulitis resulting from rheumatic fever leads to rheumatic heart disease with AS. Apart from these primary etiologies, risk factors for developing AS include aging, renal failure, hypercalcemia, smoking, metabolic syndrome, diabetes mellitus, and radiation therapy to the chest. Detection of significant AS requires detailed clinical assessment, including physical examination, echocardiography, and even cardiac catheterization. At present, applying statin therapy to treat or prevent the progression of calcific AS solely is not recommended unless there is a coexisting indication, such as atherosclerotic coronary heart disease.
Severe AS is defined as AVA ≤1.0 (N 3.0-4.0) cm2, mean gradient >40 (N <5) mmHg, or an aortic jet velocity >4.0 (N <= 2.0) M/sec. AVA <0.75 cm2 or aortic jet velocity >5 M/sec is considered critical aortic stenosis. However, clinicians should be aware that some patients with severe AS in HF with a low cardiac output state may manifest lower gradients and slower jet velocities than expected. Careful clinical assessment is essential. To differentiate actual severe from pseudo-severe AS, a low-dose dobutamine stress echocardiography might be helpful. Specifically, suppose dobutamine stress transthoracic echocardiography shows an AVA ≤1 cm2 (with AVA indexed to BSA ≤0.6 cm2/m2) with transvalvular mean gradient ≥40 mmHg (or Vmax ≥4 m/s); in that case, classical low flow low gradient severe AS is confirmed.
Indications for aortic valve replacement in adults with AS include 1. Symptomatic AS with angina, dyspnea, or syncope. 2. Asymptomatic AS with LV dysfunction (e.g., LVEF <50%) or abnormal responses to stress exercise testing (e.g., failure of the blood pressure to rise appropriately, development of arrhythmias, or the emergence of symptoms). 3. Concurrent surgery such as coronary artery bypass grafting (CABG) or other surgery for the heart or the aorta when deemed safe and necessary.
The decision-making between surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR)* is crucial. It depends on many factors, including the patient’s risk profile, comorbidities, valvular anatomy, local expertise, and preference. TAVR is a minimally invasive surgical procedure. It is mainly indicated for patients with severe AS who are at intermediate or high risk for SAVR. In recent years, TAVR has increasingly been utilized even in lower-risk patients due to comparable outcomes with SAVR. Clinically, still, others may not be able to undergo TAVR because of anatomic reasons (annulus size, vascular access, etc.), and some others due to lack of the potential benefit of TAVR owing to comorbidities such as severe COPD, end-stage renal disease, cirrhosis of the liver, etc. Given the age (92) and comorbidities (i.e., COPD, DM, liver and kidney dysfunction), it was appropriate for the care team to recommend TVAR in the present case. Lastly, balloon dilation of the aortic valve may be considered for short-term hemodynamics and clinical symptom improvement in certain patients.
*Cold agglutinin test is deemed significant at 64x+ or greater. Cold agglutinin disease (CAD) is a rare form of autoimmune hemolytic anemia. The body’s immune system abnormally produces antibodies (cold agglutinins) that attack (agglutinate) red blood cells at lower body temperatures (usually 28–31°C). The agglutinated red blood cells can lyse upon rewarming, leading to hemolysis. While the cause of primary CAD is unknown, the most common triggers of secondary CAD are infections and malignancies. Infectious causes include Mycoplasma pneumoniae, Epstein-Barr virus, cytomegalovirus, HIV, and certain bacterial infections like Legionella and Campylobacter. Malignancies, particularly B-cell lymphoproliferative disorders, like non-Hodgkin lymphoma and chronic lymphocytic leukemia, can manifest as CAD. Certain autoimmune diseases, such as systemic lupus erythematosus, can also result in secondary CAD. Symptoms for CAD provoked by exposure to cold can range from mild to severe, including fatigue, pale or yellowed skin color (jaundice), dark-colored urine, cold hands and feet, and chest pain. In extreme cases, it can lead to thrombosis, HF, and shock.
**Transudate is a fluid that leaks from blood vessels due to an imbalance in hydrostatic pressure (the force that pushes fluid out of the vessels) and osmotic pressure (the force that draws fluid into the vessels). This imbalance can be caused by conditions such as HF, cirrhosis of the liver, or nephrotic syndrome. Transudate is a clear, straw-colored fluid with a low protein content (< 25 g/L) and a specific gravity of < 1.012, low LDH level (< 60 units/L), low cholesterol content (< 45 mg/dL) and usually contains no cells (i.e., WBCs or RBCs). In contrast, exudate is a fluid that leaks from blood vessels due to inflammation or increased vascular permeability. It can be caused by pneumonia, pleurisy, or cancer. Exudate is a cloudy or purulent (pus-filled) fluid with a high protein content (> 25 g/L) and a specific gravity of > 1.020, high LDH level (> 60 units/L), high cholesterol content (>45 mg/dL), and often contains WBC and RBC blood cells.
***During TAVR, a bioprosthetic valve is delivered to the native aortic valve site via a catheter under angiographic guidance. The delivery could be performed through either a transfemoral (via the femoral artery) or transapical (direct puncture of the left ventricular apex) approach. The choice depends on patient anatomy, comorbidities, and institutional preferences. The prosthetic valve is mounted on a delivery device or ‘balloon,’ and then it is passed through the artery until it reaches the site of the old valve. The balloon is then expanded to deploy the new valve, pushing aside the native diseased valve leaflets. The new valve starts functioning immediately after deployment. The advantages of TAVR over traditional surgical replacement include Less trauma, shorter hospital stays, quicker recovery times, and potentially improved survival rates in select patients who are high-risk surgical candidates. However, potential risks are also present, including stroke, vascular complications, permanent pacemaker implantation (due to procedure-induced A-V block), and paravalvular leak. Hence, the Heart team decides on TAVR after carefully considering the patient’s situation.
Keywords:
aortic stenosis, aortic valve replacement, biventricular heart failure
References
UpToDate:
Clinical manifestations and diagnosis of aortic stenosis in adults
Indications for valve replacement for high gradient aortic stenosis in
adults
Clinical manifestations and diagnosis of low gradient severe aortic
stenosis
Medical management of symptomatic aortic stenosis
Aortic valve area in aortic stenosis in adults
Leave a comment

Case 1
A 57-year-old woman with a long-standing history of bronchial asthma and COPD was admitted because of intermittent episodes of dyspnea associated with palpitations and general
Contact
If you have further questions or have interesting ECGs that you would like to share with us, please email me.