Case 5
A 72-year-old woman, who had been in the hospital for treatment of right leg cellulitis for two days, suddenly developed increasing SOB with worsening heart
An 88-year-old woman complained of a loss of appetite, generalized weakness for two weeks, and increasing SOB for five days. She had chronic heart failure (HF), presumably caused by hypertension and diabetes mellitus, for over 30 years. She also had chronic renal dysfunction complicated by anemia. Notably, she had had worsening HF 3 times, likely caused by dietary indiscretion for the past two years, most recently five months prior. Her medications include furosemide, losartan, amlodipine, and antidiabetic medicines (metformin and insulin). On admission, she was slightly obese and in moderate respiratory distress. BP measured 163/55 mmHg, BT36.4ºC, PR 40/min, irregular, and RR 20/min. There was a notable increase in the A-P diameter of the chest with a prolonged expiratory phase, suggestive of COPD (or worsening HF). The PMI of the heart was not visible, but there were murmurs of MR (Gr3/6 and AR Gr2/6) and a few crackles at both lung bases. There was also 2+ leg edema. ECG showed LVH in sinus rhythm with 4:3 AV Wenckebach (sinus rate 65/min, ventricular rate 42/min). Chest X-ray revealed a calcific aortic knob, probable LV and LA enlargement, possible COPD, and prominent PA suggestive of pulmonary hypertension. Pertinent laboratory data included Hb 9.8 gm/dL, WBC 3600, Plt 170 K/ul, SGOT 18 IU/L, BNP 815 pg/mL, BUN 36 mg/dL, Cr 3.1 mg/dL, Na 142 mEq/L, K 3.3 mEq/L, CKMB 2.0 ug/L, troponin-I 0.03 ug/L, CRP 0.4 (N <3) mg/L, Glucose AC 133 mg/dL. The care team recognized the worsening of HF and the progression of the first-degree to second-degree A-V block over five months. They elected not to repeat echocardiography with Doppler as it had shown enlarged LA and concentric hypertrophy of LV with an ejection fraction (EF) of 58% and predominantly diastolic dysfunction, consistent with HFpEF* five months prior. The estimated systolic PA pressure (RVSP) was 57 mmHg. They ascribed the progression of conduction disturbance to her age and associated cardiomyopathy. In two days, they decided to implant a dual-chamber permanent pacemaker (DDD mode, rate: 60-130).
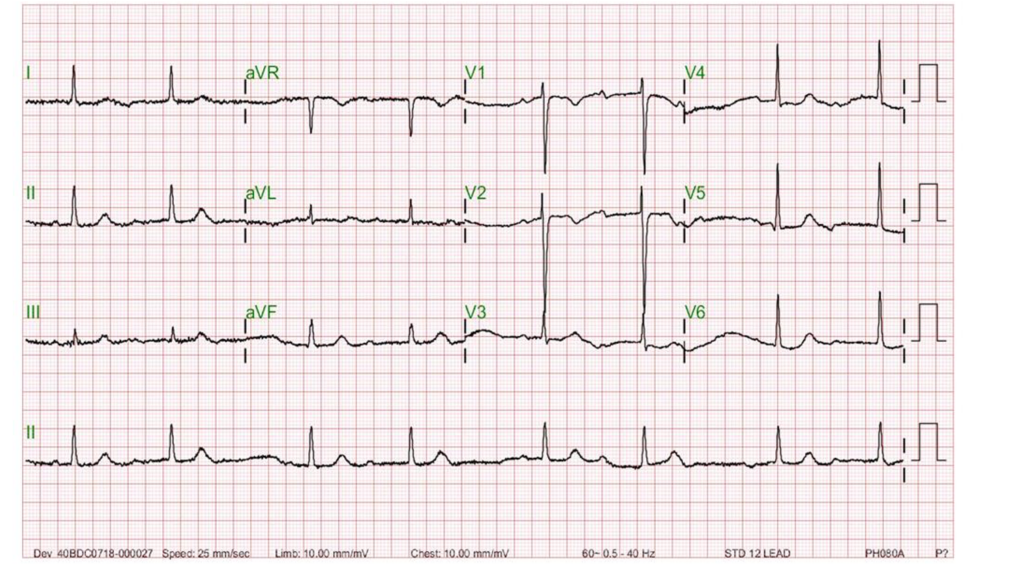
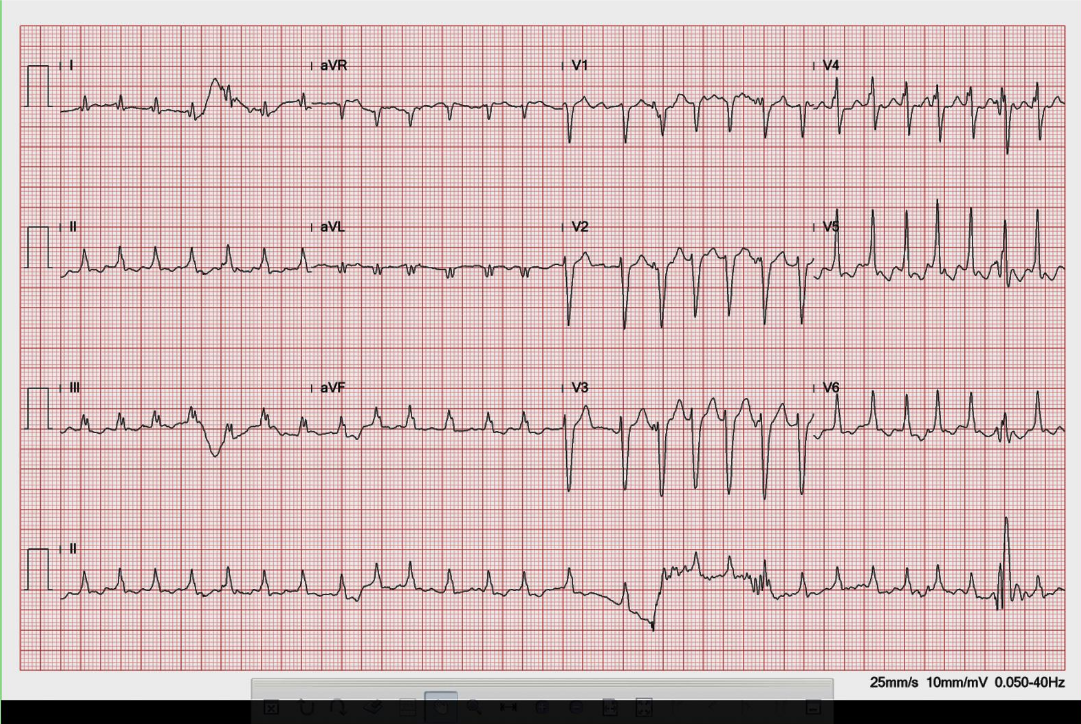
Sinus rhythm with 3:2 AV Wenckebach (Mobitz I); sinus rate 70/min.
LVH
Unstable baseline
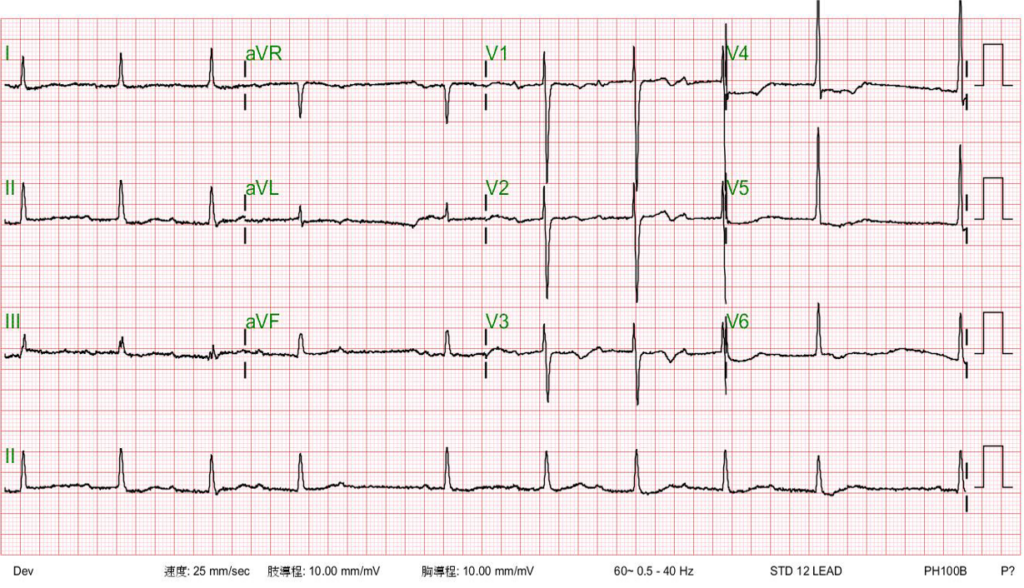
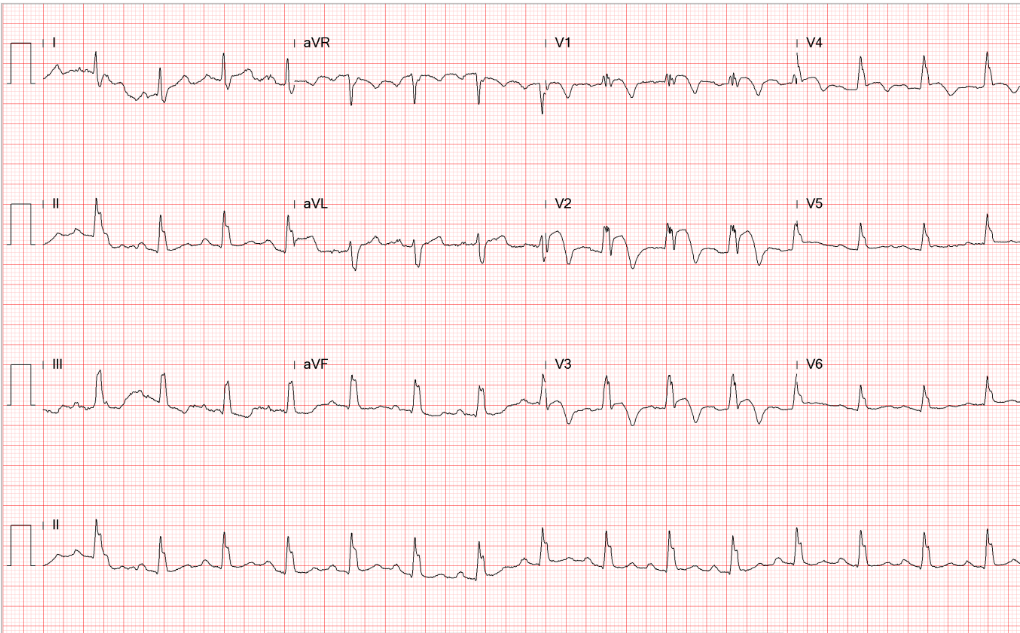
Sinus rhythm with 4:3 AV Wenckebach (sinus rate 65/min)
LVH with ST-segment depression in V4-V5 and nonspecific T changes.
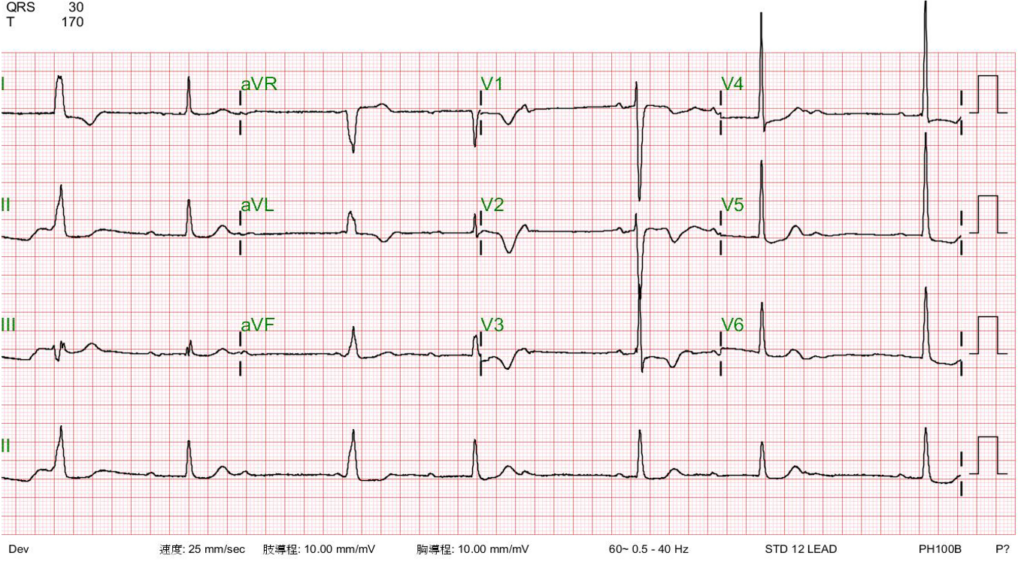
Sinus rhythm with AV Wenckebach (sinus rate 65/min) interfered by a ventricular escape mechanism
LVH with ST-segment depression in V4-V6 and nonspecific T changes.
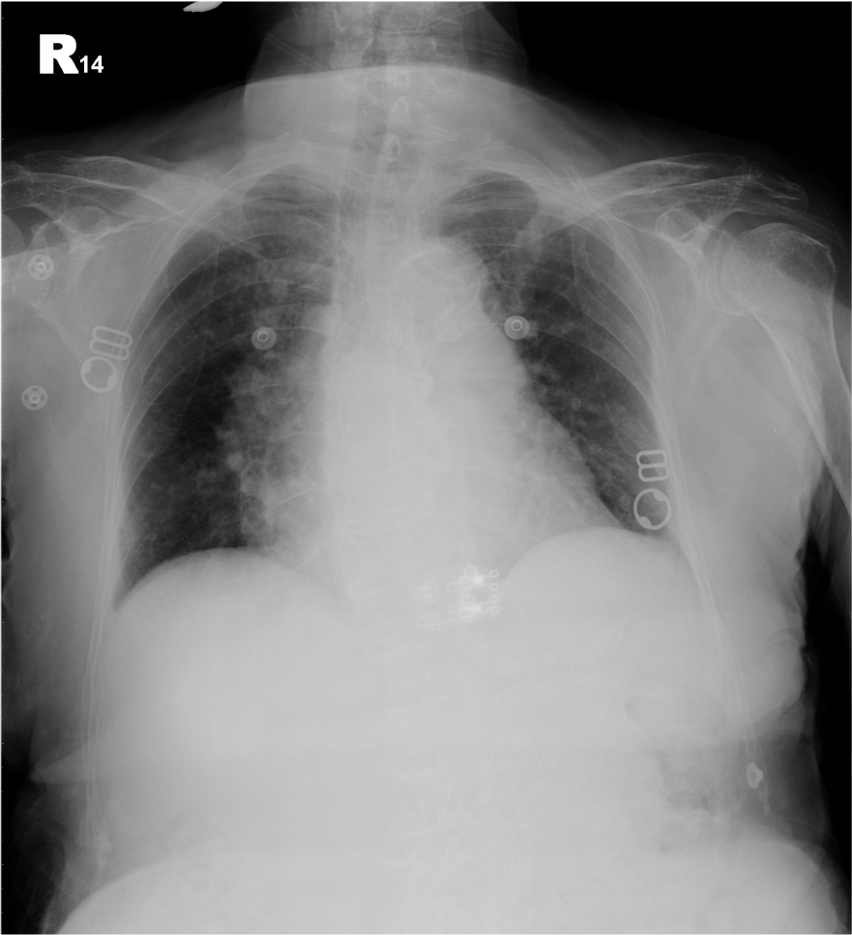
Increased cardiothoracic ratio
Probable LV and LA enlargement
Calcific aortic knob
Prominent pulmonary arteries suggestive of pulmonary hypertension
Possible COPD
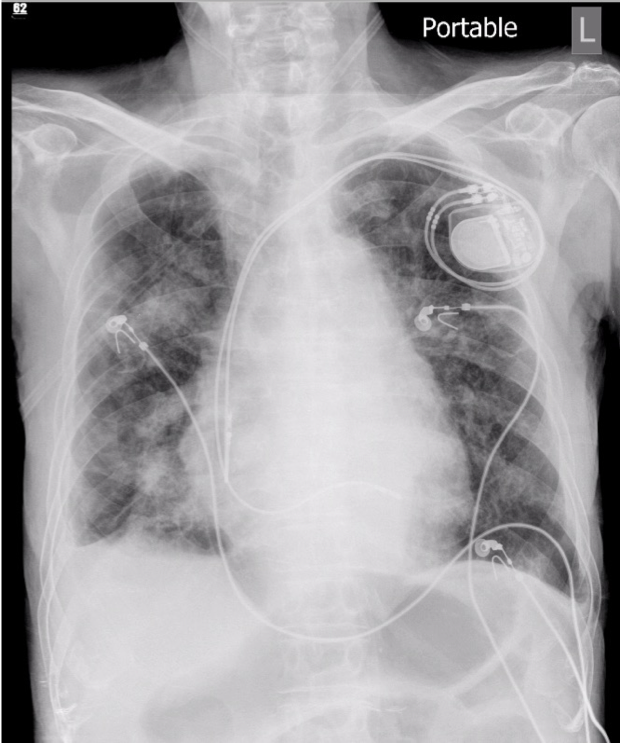
Progressive infiltrates at bilateral lung fields compared with the previous study
A dual-chamber pacemaker in place
A-V Wenckebach block, also known as Mobitz I A-V block, is characterized by a progressive lengthening of the PR interval until a beat is dropped on ECG.** This type of A-V block is usually indicative of A-V nodal dysfunction. Thus, it is generally benign and does not typically cause significant symptoms or complications. It is often seen in younger individuals and may be associated with underlying conditions such as enhanced vagotonia, medications (e.g., beta-blockers), and febrile illnesses. On the other hand, Mobitz II AV block is characterized by intermittent failure of A-V conduction without progressive PR interval prolongation. This type of A-V block is caused by disease occurring at the His bundle and is often associated with bundle branch block. As such, it is more concerning, as it is more likely to progress to complete the A-V block. Moreover, Mobitz II AV block is often associated with structural heart disease, such as myocardial infarction or cardiomyopathy. It usually requires permanent pacemaker therapy.
In A-V Wenckebach (Mobtz type 1), the degree of the block is determined by the number of P waves before the block. The pattern of 3-to-2 block, as shown in the first ECG, is the most common. When the 4th P wave is blocked, illustrated in the second ECG, it is referred to as a 4-to-3 block, which is less common. The pattern of 5-to-4 block is very uncommon. When encountering a 2-to-1 A-V block pattern, differentiating between Mobitz type 1 and Mobitz type 2 can be difficult. However, some differences might be observed. If the PR interval is already prolonged, Mobitz type 1 is more likely, and if the QRS complex is abnormal, like RBBB or LBBBB, Mobitz type 2 block is more likely.
Nevertheless, the clinical significance of Mobitz I and Mobitz II A-V block depends on several factors, including the frequency and severity of symptoms, the underlying cause, and any associated cardiac conditions. Symptoms of A-V block can range from mild, such as dizziness or fatigue, to more severe, such as syncope (fainting) or hemodynamic instability. In medicine, there are exceptions to the rules. Given the patient’s age and the extreme nature of HF-associated multiple comorbidities (i.e., diabetes, hypertension, cardiomyopathy, COPD, and renal dysfunction), pacemaker therapy is the correct choice for this patient despite A-V conduction disturbance being Mobitz type I (Wenckebach) A-V block.
Elevation of BNP (815 pg/mL) supports the diagnosis of HF. The patient likely has biventricular failure due to pulmonary hypertension and leg edema. The Myocardial stretch is one of the most critical stimuli of BNP and NT-proBNP release from the heart. For people who don’t have HF, normal BNP levels are less than 100 pg/mL. For NT-proBNP, normal levels are less than 125 pg/mL for people under 75 years old and less than 450 pg/mL for people over 75. BNP over 100 pg/mL or NT-proBNP levels over 900 pg/mL may indicate HF. The kidney excretes BNP and NT-proBNP levels; their values are higher in renal dysfunction patients. However, clinicians should also realize that patients with renal dysfunction often have significant cardiovascular diseases that cause elevated BNP and NT-proBNP. BNP and NT-proBNP can become elevated in conditions other than HF, like acute coronary syndrome, myocarditis, valvular heart disease, hypertrophic cardiomyopathy, cardiotoxic drugs, atrial fibrillation or flutter, RV dysfunction in the setting of significant pulmonary disease (e.g., pulmonary hypertension [as in the present case], pulmonary embolism), etc.
The symptomatology described by the patient (i.e., a loss of appetite, generalized weakness, and increasing SOB) resonates with worsening HF. Cardiac arrhythmias, both bradycardia (such as A-V Wenckebach as in the present case) and tachycardia (e.g., AF) can both worsen HF through different mechanisms. Bradycardia can reduce cardiac output and impair the delivery of oxygen and nutrients to the body’s tissues. In HF, where the heart cannot pump blood effectively, bradycardia can further decrease cardiac output and exacerbate symptoms. Additionally, bradycardia can cause blood to pool in the heart chambers, increasing the risk of blood clots and stroke.
On the other hand, tachycardia can also worsen HF. During tachycardia, the heart has less time to fill with blood between contractions, reducing the amount of blood pumped out with each heartbeat and decreasing cardiac output. Tachycardia can also increase the workload on the heart, causing it to work harder. Moreover, persistent tachycardia can lead to electrical remodeling of the heart, the so-called tachycardia-induced cardiomyopathy, compromising cardiac function. Thus, both bradycardia and tachycardia can worsen HF. It is essential to manage heart rate in patients with heart failure to optimize cardiac function and improve symptoms.
Other factors besides cardiac arrhythmias can worsen the condition in patients with chronic HF. These factors can include but are not limited to: 1. Non-adherence to medication and treatment plan. 2. Dietary indiscretion causing fluid and sodium retention. 3. Poorly controlled hypertension: 4. Acute myocardial ischemia/infarction 5. Anemia, 6. Medication side effects or interactions, like nonsteroidal anti-inflammatory drugs (NSAIDs) or certain antiarrhythmic drugs. 7. Infections such as pneumonia. 8. Renal dysfunction 9. Emotional stress and depression, etc. Patients need to work closely with their healthcare provider to identify and manage these factors to optimize their HF management and improve their quality of life. Lastly, multiple comorbidities are usually present in older adults, which may contribute to HF development or worsening.
In the present case, the care team needs to work on other issues to improve HFpEF management, in addition to pacemaker therapy, especially hypertension (163/55 mmHg on admission), anemia (Hb 9.8 gm/dL), renal failure (BUN 36 mg/dL, Cr 3.1 mg/dL), and COPD. With diabetes, hypertension, and chronic kidney disease, the care team should consider the best treatments that reduce CKD progression (e.g., renin-angiotensin-aldosterone system [RAAS] inhibition and SGLT2 inhibitor therapy). Lastly, carefully selecting drugs, such as beta-blockers, is mandatory because of COPD.
*Classification of HF based on LV EF:
Remember that each patient’s situation is unique, and treatment should be tailored accordingly.
**There are two descriptive terms for the A-V Wenckebach phenomenon: typical and atypical. In the typical form, the R-R interval gets shorter before the dropped beat (non-conducted P wave) because the increment of the P-R prolongation gets less and less. On the other hand, in the atypical form, the R-R interval gets longer when the increment of the P-R prolongation progressively increases. During A-V Wenckebach, ventricular the ventricular escape mechanism may emerge due to slow ventricular rate (e.g., ECG 3), mimicking complete A-V block. These ECG findings are interesting but bear no further clinical significance as they all indicate A-V nodal dysfunction. The atypical form may occur more commonly than the typical form.
****Another descriptive term relating to Wenckebach is “alternating Wenckebach periodicity.” It is defined as an episode of 2:1 A-V block with progressive lengthening of the conducted P-R intervals leading to two or three blocked P waves (i.e., 3-to-1, 4-to-1 block), the so-called high degree A-V block. This unique phenomenon may reflect two levels of conduction block in the cardiac tissues, including the atrium, A-V node (with or without horizontal electrophysiologic dissociation), His-Purkinje system, and even the accessory pathway. A high atrial pacing rate can create this high-degree A-V block pattern. However, when clinically seen, it may be associated with drug toxicity, such as digoxin with quinidine. It is essential to have a high degree of suspicion, as the patient may require immediate treatment or pacemaker therapy.
Keywords:
A-V Wenckebach phenomenon, second degree A-V block (Mobitz I and Mobitz II), BNP and NT-proBNP
UpToDate:
ECG tutorial: Atrioventricular block
Natriuretic peptide measurement in heart failure
Heart failure: Clinical manifestations and diagnosis in adults
Treatment and prognosis of heart failure with preserved ejection
fraction
Lam CSP and Solomon SD. Classification of heart failure according to ejection fraction: JACC review topic of the week J Am Coll Cardiol. 2021 Jun 29;77:3217-3225

A 72-year-old woman, who had been in the hospital for treatment of right leg cellulitis for two days, suddenly developed increasing SOB with worsening heart
This 43-year-old woman was rushed to ER because of the sudden onset of substernal chest tightness accompanied by cold sweats, which lasted more than 30
An 81-year-old man came to the Emergency Department (ED) after one day of having progressive dyspnea with orthopnea and had diffuse itchy skin for more
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.