Case 17
A 66-year-old woman was admitted with chest tightness and intermittent palpitations at rest for one week. Past medical history is significant for hypertension and chronic
A 41-year-old Taiwanese woman, G3P1, developed progressive dyspnea on exertion, PND, and orthopnea for five days after she gave birth to a healthy baby boy (vaginal delivery) three months prior. Notably, she had preeclampsia manifesting as dizziness, nausea, vomiting, abdominal pain, diarrhea associated with moderate hypertension, and proteinuria during the 3rd trimester. On arrival at the emergency room, she was afebrile and in mild respiratory distress; BP measured 88/66 mmHg and BT/PR/RR 37℃, 100/min, regular, and 18/min, respectively. On physical examination, the heart was enlarged, JVD (+12 cm H2O) was visible, and there were audible S4 and S3 but no significant murmurs and coarse crackles in both lungs. Leg edema was 2+. ECG showed diffuse low voltage in sinus tachycardia at 107/min, regular, with nonspecific ST-T abnormalities. Chest X-ray revealed cardiomegaly with pulmonary congestion indicative of heart failure (HF). Pertinent laboratory data included Hb 13.0 g/dL, WBC 6500, Plt 183 103, SGOT 71 (N15-41) IU/L, SGPT 96 (N 7-56) IU/L, and troponin-I 0.03 (N <0.05) ng/mL, CRP 0.63 (N <81) mg%, Na 139 mEq/L, K 4.5 mEq/L, Cr 1.0 mg/dL, BUN 21 mg/dL, BNP 560 (N <100) pg/ml, TSH 1.2 (N 0.4-4.2) mIU/L, free T4 (N 0.8-1.8) ng/dL. Echocardiograms confirmed LV enlargement with impaired global contractility (LV EF 27.1 %), so did the cardiac MRI. As no apparent etiology (e.g., long-standing hypertension or atherosclerotic coronary heart disease [CHD] with previous MI) could be found, the care team suspected peripartum cardiomyopathy (PPCM) and started treatment for biventricular HF with intravenous furosemide, oral losartan, spironolactone, and aspirin. She gradually improved and was discharged with a compromised but improved LV function (EF 37.2% entering the class of HFimpEF*) in 3 weeks.
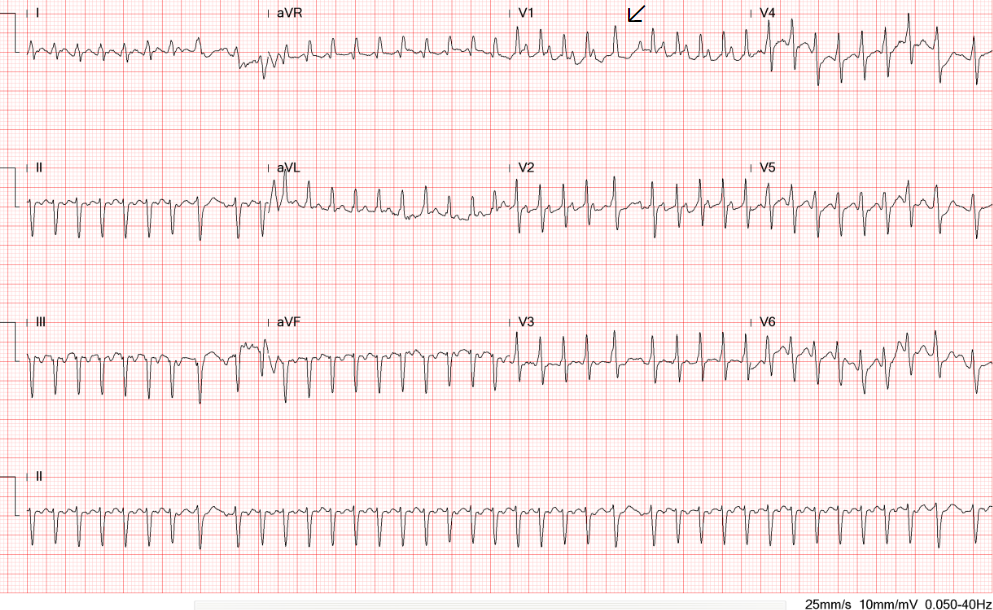
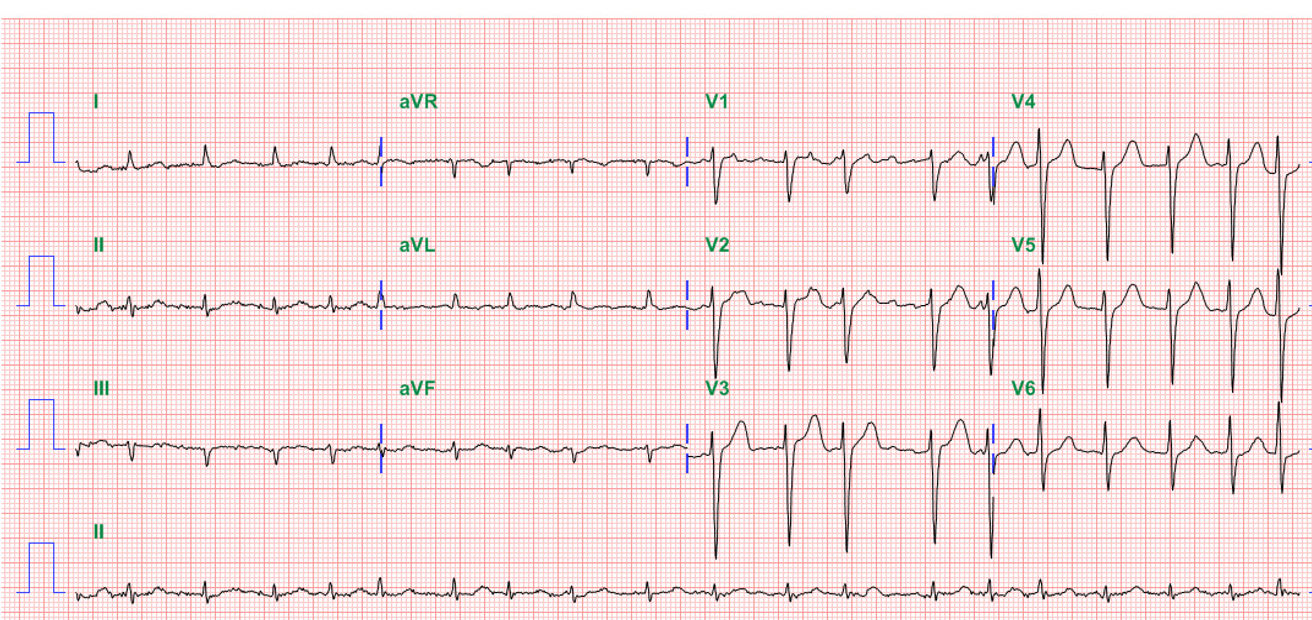
Diffuse low voltage
Sinus Tachycardia at 107/min with nonspecific ST-T changes
Borderline 1st degree A-V block (P-R 210 msec)
Left axis deviation (QRS axis -47°) with slow progression of R wave in V1-V5
QT prolongation (QTc 495 msec)
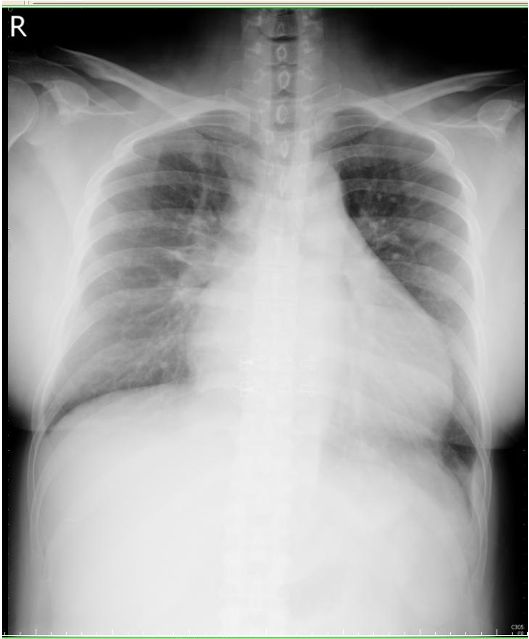
Cardiomegaly
Increased bilateral lung markings consistent with pulmonary congestion
Blunting of left costophrenic angle indicating pleural effusion.
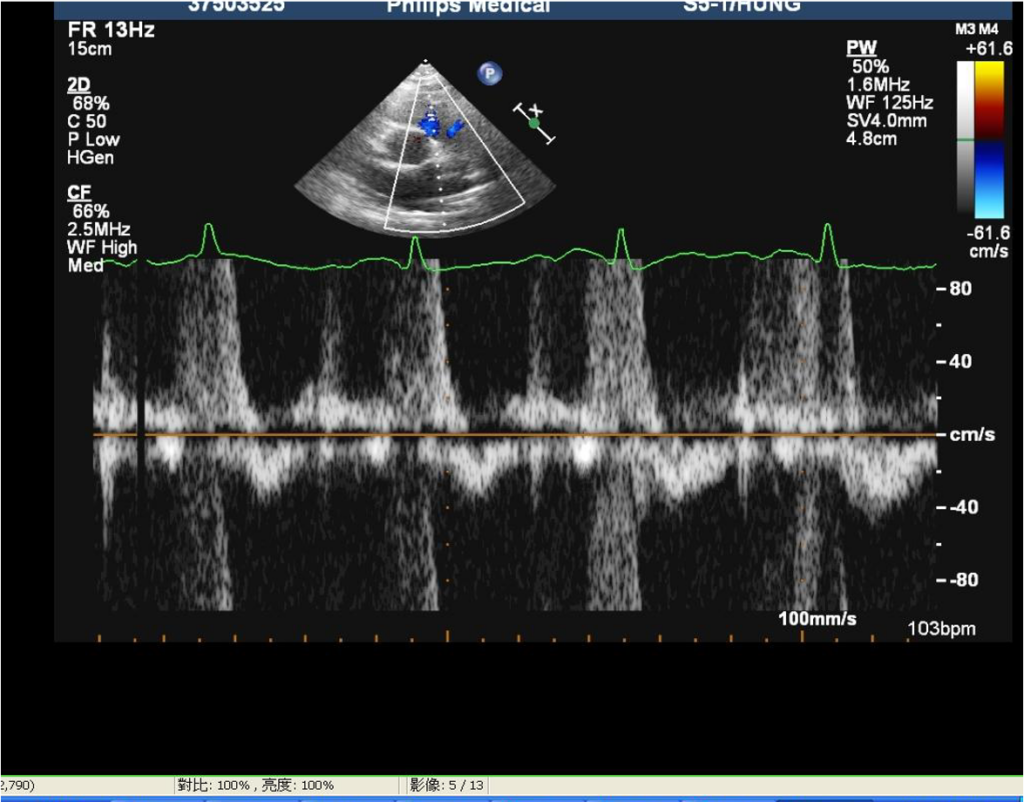
LV enlargement.
Impaired global contractility of LV (EF 27.1 %)
Mild AR, MR PI, and TR
ECG findings of sinus tachycardia with ST-T changes and borderline 1st-degree A-V block are nonspecific. In the present case, the diffuse low voltage with a left axis should consider obesity, pericardial effusion, and myocarditis/cardiomyopathy as the cause. Moreover, the associated QT prolongation with QTc of 495 msec should arouse attention to whether hypokalemia or hypomagnesemia is the culprit and correct it accordingly while managing HF. QT prolongation can lead to life-threatening ventricular tachyarrhythmias such as Torsade des Pointes, degenerating into ventricular fibrillation and causing sudden death.
Physical findings and results of diagnostic work-ups indicate this is a case of HF with reduced EF (HFrEF)* associated with a form of dilated cardiomyopathy. Right-sided HF resulting from Left-sided HF is likely the cause of JVD (12 cm H2O), elevated liver enzymes (SGOT 71 IU/L, SGPT 96 IU/L)(liver congestion r/o viral hepatitis), and leg edema. Based on the present illness and past medical history, the care team reasonably suspects PPCM by exclusion, as no other causable diseases (e.g., hypertension, CHD, etc.) are attributable. Other considerations should include the hereditary form of dilated cardiomyopathy and cardiomyopathy related to myocarditis and endocrine disorders (e.g., hyper- and hypothyroidism). PPCM is an independent entity distinct from other cardiomyopathies. However, specific biomarkers to distinguish between PPCM and other cardiomyopathies remain unavailable.
PPCM is a rare but devastating form of HF that occurs toward the end (usually the last month) of pregnancy or in the months (usually five months) following delivery when no other cause of HF can be found. Research suggests that hemodynamic alteration, oxidative stress, preeclampsia, cardiotropic viruses, hormonal and immune system dysfunction, including autoimmunity, deficiencies in specific nutrients, such as selenium or vitamin D, pre-existing cardiovascular risk factors (hypertension, diabetes, or obesity), and genetics (e.g., black, STAT3 deficiency) are likely contributing factors.
Although there is no direct link between gene STAT3 defect and PPCM, studies have shown that deregulated STAT3 circuits are sufficient to induce dilated and peripartum cardiomyopathy in mice. A combination of increased oxidative stress during late gestation and the early postpartum period and high levels of the nursing hormone prolactin are likely a crucial pathophysiological etiology of PPCM. Elevated oxidative stress protects against infection, but sensitive organs like the heart need antioxidants to counteract oxidative stress to avoid oxidative damage.
Without STAT3, there is an increase in the enzyme cathepsin D, which clips prolactin hormone to a shorter form, 16 kDa prolactin, that can block blood vessel formation and cause cardiac cells to become oxygen-deprived and die. Consequently, the heart’s pumping action fails and clinically develops HF. STAT3 is a signaling molecule and transcription factor that plays critical protective roles in the heart. It is suggested to function protectively in mitochondria, where it regulates reactive oxidative spices (ROS) production, partly by regulating the activities of the electron transport chain complexes. STAT3 is also a key player known to be activated in the cardioprotective ischemic conditioning protocols. Through these varied roles, STAT3 participates in various mechanisms contributing to cardioprotection against different heart pathologies, including PPCM.
Managing acute PPCM involves a combination of medical therapies and supportive care. 1. Medications such as diuretics (to reduce fluid retention), beta-blockers (to control heart rate and blood pressure), angiotensin receptor neprilysin inhibitor (to reduce afterload), and a mineralocorticoid receptor antagonist (to reduce inflammation), and in some cases, anticoagulants (to prevent blood clots). 2. Supportive measures include close monitoring of vital signs, oxygen therapy, fluid and sodium intake restriction, and managing any associated complications, such as arrhythmias or thromboembolic events. 3. After stabilization of the acute phase, cardiac rehabilitation programs help improve cardiac function, physical fitness, and overall well-being. 4. In cases where medications prescribed for PPCM may interfere with breastfeeding, discussions with healthcare providers regarding the risks and benefits of breastfeeding should be undertaken. It’s important to note that the management of PPCM should be individualized based on the patient’s specific clinical presentation and needs. Close collaboration between the patient’s obstetrician, cardiologist, and other healthcare providers is essential for optimal care.
Bromocriptine, a dopamine receptor agonist not tried in the present case, is a clinically-approved drug that blocks prolactin release. It works by inhibiting prolactin release, thereby reducing myocardial inflammation. It has been tested in a mouse model of peripartum cardiomyopathy (PPCM), where it efficiently prevented the onset of PPCM. This treatment concept was transferred to humans as a bench-to-bedside approach and successfully used in humans. The so-called BOARD concept comprises bromocriptine, oral heart failure therapy, anticoagulation, vaso-relaxing agents, and diuretics).
In acute PPCM with cardiogenic shock, levosimendan** or temporary circulatory support devices should be used, and catecholamines such as dopamine should be avoided. In contrast to other forms of cardiomyopathies, such as dilated cardiomyopathy, most PPCM patients have a high potential for partial or even full recovery if early and optimal treatment is applied. Suboptimal treatment may worsen the disease and lead to irreversible heart failure.
*New classification of HF by LV ejection fraction (EF):
HF with reduced EF (HFrEF) – symptomatic HF with LVEF ≤40%
HF with mildly reduced EF (HFmrEF) – symptomatic HF with LVEF 41-49% (previously labeled as HF with mid-range EF)
HF with preserved EF (HFpEF) – symptomatic HF with LVEF ≥50%
HF with improved EF (HFimpEF) – a new classification which is distinctly defined as symptomatic HF with a baseline LVEF ≤40%, a ≥10-point increase from baseline LVEF, and a second measurement of LVEF >40%
(Gibson G et al. From universal definition and classification of heart failure: A step in the right direction from failure to function. J Amer Coll Cardiol Jul 13, 2021)
**Levosimendan is a distinctive inodilator that combines calcium sensitization, phosphodiesterase inhibition, and vasodilating properties by opening adenosine triphosphate-sensitive potassium channels. It was first approved for clinical use in 2000 in Sweden, followed by other European countries. Now, it is actively tried in the US.
Keywords:
bromocriptine, peripartum cardiomyopathy, prolactin, reactive oxidative spices
UpToDate: Peripartum cardiomyopathy: Etiology, clinical manifestations, and
diagnosis
Peripartum cardiomyopathy: Treatment and prognosis
Hilfiker-Kleiner D et al. A cathepsin D-cleaved 16 kDa form of prolactin
mediates postpartum cardiomyopathy. Cell 2007; 128:589–600.
Koenig T et al. Bromocriptine for the treatment of peripartum cardiomyopathy.
Cardiac Failure Review 2018;4:46–9.

A 66-year-old woman was admitted with chest tightness and intermittent palpitations at rest for one week. Past medical history is significant for hypertension and chronic
An 84-year-old man with a known history of hypertension, COPD, diabetes, Parkinsonism, and old CVA (putamen infarction)* was noted to be delirious after five days

An 83-year-old woman was transferred to Emergency Department (ED) from a local hospital because of a marked deterioration of general status while being treated with
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.