Case 2
A 58-year-old man, who had been in good health, became infuriated after a quarrel with his wife for 20 minutes. He suddenly experienced severe chest
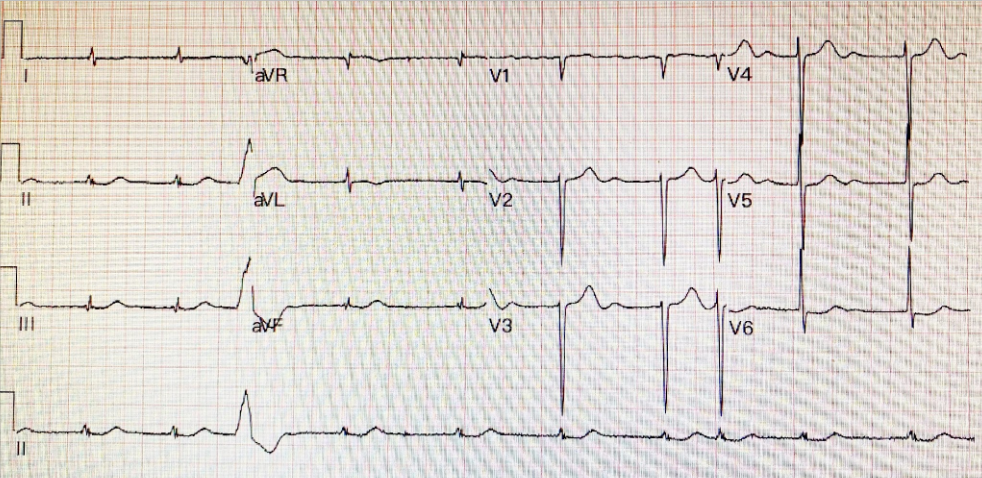
A 75-year-old man complained of worsening dyspnea with orthopnea for 2 hours. He had no chest pain or palpitations. He admitted dietary indiscretion and noted a gradual weight gain of about 12 lbs in the past two months. He was brought to the Emergency Department (ED) as advised by his physician. Past medical history was significant for chronic heart failure (HF) due to hypertensive cardiomyopathy, atrial fibrillation (AF), and chronic renal dysfunction related to polycystic kidney disease and diabetic nephropathy over 30 years. Reportedly, he had worsened HF with similar symptomatology twice before, and a diagnostic workup with a persantin thallium stress scan was negative for significant myocardial ischemia one year prior. Medications included furosemide, apixaban, atenolol, digoxin, valsartan, metformin, and glipizide. He was in acute respiratory distress at ED with RR 24/min, BP 160/65 mmHg, and PR 85/min, irregular. Physical examination revealed no JVD or leg edema but notably for displaced PMI (the 5th intercostal space at LAAL), an apical Gr III/IV systolic murmur, and bilateral crackles at both posterior lung fields. After a blood draw for laboratory study, he suddenly collapsed and lost consciousness. At the same time, an ECG showed wide QRS complex tachycardia recognized as ventricular in origin at a rate of 209/min. Accordingly, he received a synchronized 100-joule biphasic shock to convert the VT. He regained consciousness and was admitted for treatment of worsening HF. ECG during baseline showed AF with LVH at 63/min. The QTc interval measured 480 msec. Pertinent laboratory data included Hb 11.8 gm%, WBC 6800, AST 24 (N 8-48) IU/L, ALT 28 (N 7-55) IU/L, CRP 1.6 (N <0.3) mg/dL, Na 134 mEq/dL, K 3.2 mEq/dL, BUN 57 mg/dL, Cr 3.4 mg/dL CKMB 16 (N 5-25) IU/L, troponin-I 0.02 (N 0-0.04) ng/ml, BNP 486(N <100) pg/ml and NT-proBNP 3650 (N <500) pg/mL. After diuresis, K supplement, and supportive therapy, he gradually improved. The care team emphasized the importance of dietary compliance and recommended implantable cardioverter/defibrillator (ICD) implantation.
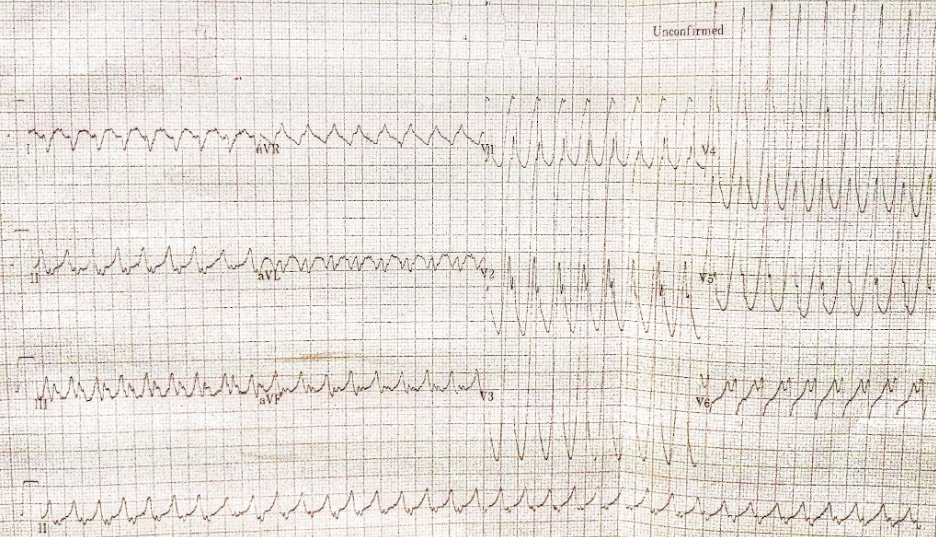
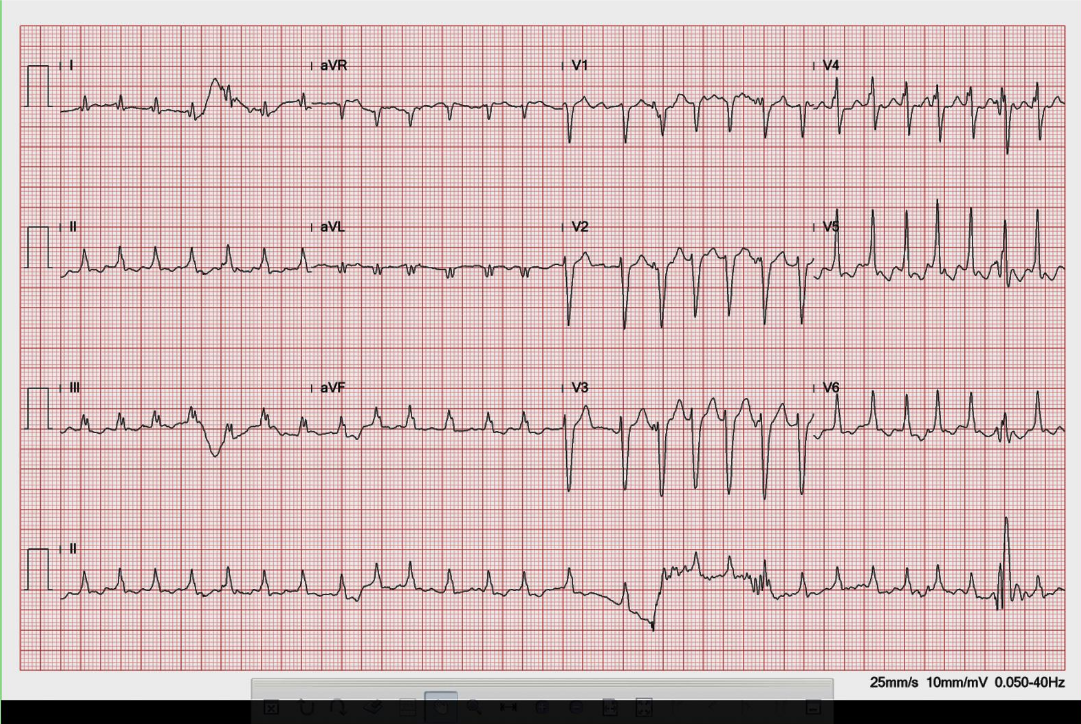
Wide QRS complex tachycardia with RBBB-like and right axis pattern (QRSd 170ms) at 209/min
Concordant QRS complexes in V1-V5
P waves not discernible
Findings consistent with ventricular tachycardia or atrial tachycardia with ventricular preexcitation at the LV lateral wall
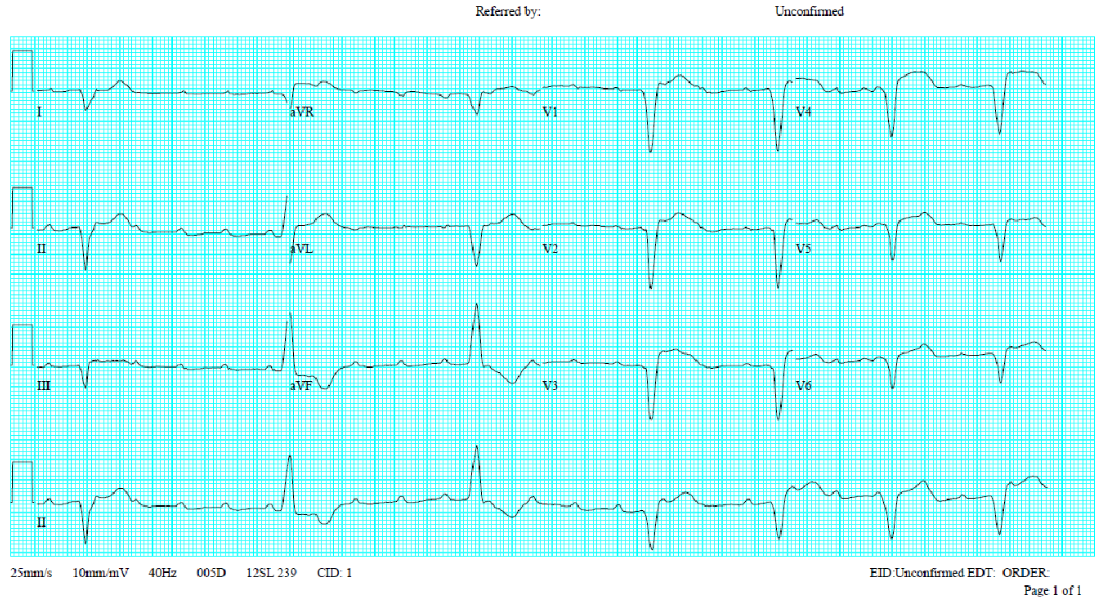
Atrial fibrillation with a ventricular rate of 63/min
LVH
QT/QTc 470/480 msec
PVC
AF with variations of measurements
Concentric LVH
LV systolic function moderately reduced with an EF of 39 %
Dilated LV, LA, and RA
Mild AI, moderate MR, and mild TR
Small Pericardial effusion
Non-sustained VT during the examination
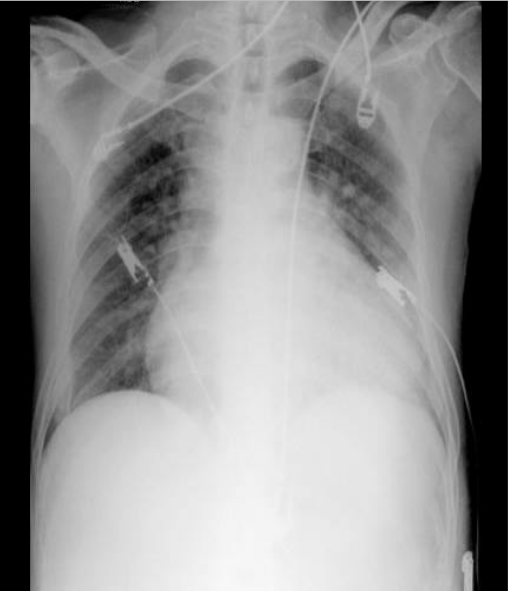
Cardiomegaly with LV, RA, and LA enlargement
Increased pulmonary vasculature suggestive of pulmonary hypertension and congestion
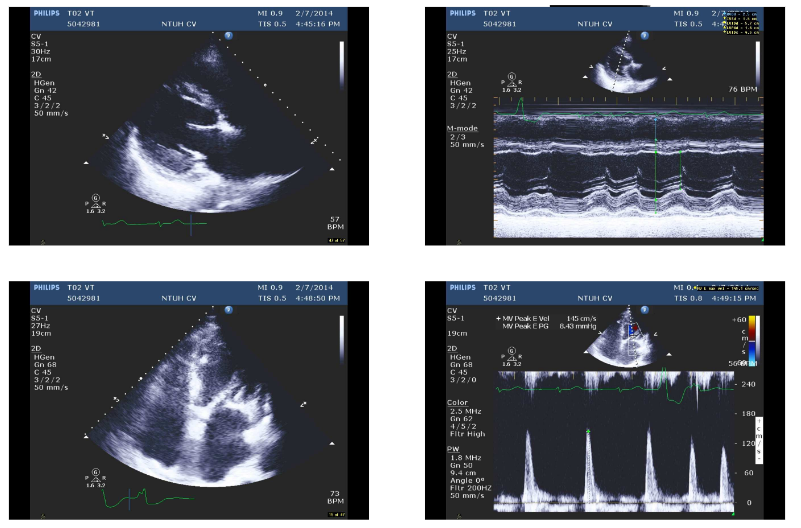
AF with variations of measurements
Concentric LVH
LV systolic function moderately reduced with an EF of 39 %
Dilated LV, LA, and RA
Mild AI, moderate MR, and mild TR
Small Pericardial effusion
Non-sustained VT during the examination
When dealing with a wide QRS complex tachycardia, differentiation between VT and supraventricular tachycardia (SVT) with aberrant conduction can be a clinical challenge. Patients with VT are usually older and have associated structural heart disease. In contrast, those with SVT are younger and have no structural heart disease. Moreover, hemodynamically, VT originates directly from the ventricle. A fast VT can readily lead to hypotension, making a patient become pulseless, lose consciousness, and even degenerate into ventricular fibrillation (VF), causing sudden death. Unfortunately, exceptions to these rules do exist.
From the ECG standing point of view, the following criteria are in favor of VT rather than SVT with aberrant conduction:
Based on the clinical context and the above criteria, we can easily recognize VT as it occurs in the present case. However, strictly speaking, since A-V dissociation is not discernable, the VT QRS morphology is also compatible with ventricular preexcitation (the Wolff-Parkinson-White syndrome) with a left lateral A-V bypass tract during an antidromic A-V reciprocating tachycardia or atrial tachycardia with 1:1 A-V conduction. Regardless, hemodynamically, ventricular preexcitation due to atrial tachycardia with 1:1 A-V conduction or antidromic A-V reciprocating tachycardia is similar to VT. Thus, the differential diagnosis for a wide QRS complex tachycardia, besides VT, includes SVT with aberrancy, preexcited supraventricular tachycardia, and antidromic A-V reciprocating tachycardia.
When encountering sustained monomorphic VT, vital signs are critical for determining the management strategy. In patients with unstable hemodynamics, such as hypotension, chest pain, altered mental status, or heart failure, as shown in the present case, immediate electrical cardioversion is indicated. Since VT is a relatively regular tachycardia, electrical cardioversion using 100-joules (at least 50-joules) biphasic or 200-joules monophasic shock* should suffice. However, it should be synchronized to the QRS complex for fear of provoking VF. If the first shock is unsuccessful, the energy level should be increased on subsequent shocks. When encountering VF, use the maximal energy, usually 200-joules biphasic or 360-joules monophasic shock, for defibrillation, and it should be non-synchronized as the fibrillatory signals of VF are too low to trigger the shock delivery. If the first shock at 200 joules (default initial energy for VF) fails, the subsequent shocks can be either the same (200 joules) or as high as 360 joules of biphasic shock.
In VT patients with stable hemodynamics, the following antiarrhythmic agents might be helpful, with hypotension being the primary adverse side effect.:
Among the causes of worsening HF, dietary indiscretion is the most commonly seen, as illustrated in this case. Other causes include myocardial ischemia or MI, cardiac arrhythmias (tachycardia or bradycardia), medical nonadherence, infection, anemia, uncontrolled hypertension, adverse effects of medications (e.g., beta-blockers, calcium-channel blockers, antiarrhythmic drugs, NSAIDs, anti-TNF antibodies), worsening renal dysfunction, etc.
The patient has compromised LV function (EF 39%) with HF and renal dysfunction (BNP 486 pg/ml, NT-proBNP 3650 pg/mL, BUN 57 mg/dL, Cr 3.4 mg/dL). For worsening HF, both acute and chronic cardiorenal syndrome and aging should also be considered. In the present case, diabetes mellitus and polycystic kidney disease further complicate the issue. Cardiorenal syndrome results from the interaction between the heart and kidney during acute and chronic HF. The common pathophysiology involves hemodynamic, neurohormonal, and inflammatory mechanisms (see Reference). Under this circumstance, the care team should consult renal and diabetic specialists if initiating an aldosterone antagonist or a sodium-glucose cotransporter-2 (SGLT-2) inhibitor might help avert the progression of HF and renal dysfunction. Otherwise, renal replacement therapy, such as intermittent hemodialysis, may become necessary.
Lastly, the patient already has three hospitalizations for worsening HF within one and a half years; the decision to implant an ICD is reasonable despite VT being the first event. Moreover, catheter ablation of the monomorphic VT might be advisable if it frequently recurs, triggering electrical shocks.
*The energy of a defibrillator is defined in joules. Joules (J) is the unit of measurement associated with one Amp of current passing through 1 Ohm of resistance for 1 second, i.e., Joules (Energy) = Time × Voltage × Current.
The main difference between monophasic and biphasic shock delivery is that a monophasic electrical current moves in a single direction. In contrast, a biphasic current is bidirectional (moving in a straight line and then reversing its trend). As such, biphasic defibrillation uses a different waveform to deliver energy to the heart in a more targeted and efficient manner, allowing for successful defibrillation at a lower energy level.
The joules of monophasic shocks are higher than those of biphasic. A monophasic shock typically has a sequence of 200- 360J, and a biphasic shock is much lower, between 120-200J. Generally, biphasic shocks are more effective for endocardial defibrillation than monophasic shocks. For transthoracic ventricular defibrillation, biphasic and monophasic shocks are equally effective, but biphasic shocks require less energy for the same efficacy. Lastly, biphasic defibrillators are now the industry standard; they are smaller and lighter than monophasic defibrillators, and they are more effective in mitigating skin burns and injury to the heart due, in part, to biphasic waveforms delivering significantly less current.
Keywords:
electrical cardioversion, supraventricular tachycardia,ventricular preexcitation syndrome, ventricular tachycardia
UpToDate:
Ventricular arrhythmias: Overview in patients with heart failure and cardiomyopathy
Sustained monomorphic ventricular tachycardia in patients with structural heart disease: Treatment and prognosis.
Basic principles and technique of external electrical cardioversion and defibrillation
Rangaswami J et al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association.Circulation. 2019;139:e840–e878

A 58-year-old man, who had been in good health, became infuriated after a quarrel with his wife for 20 minutes. He suddenly experienced severe chest

A 65-year-old man was brought in with progressive edema of the left arm below the elbow level for two weeks. It was not “red and
A 72-year-old woman, who had been in the hospital for treatment of right leg cellulitis for two days, suddenly developed increasing SOB with worsening heart
If you have further questions or have interesting ECGs that you would like to share with us, please email me.
©Ruey J. Sung, All Rights Reserved. Designed By 青澄設計 Greencle Design.