An Uncharted Journey

By Ruey Jen Sung (宋瑞珍)
Professor Emeritus of Medicine
Cardiovascular Medicine, Department of Medicine
Stanford University School of Medicine
Biographical sketch
Dr. Ruey Jen Sung is a Professor Emeritus of Cardiovascular Medicine at Stanford University and the University of California, San Francisco (UCSF). From 1991 to 2001, he served as the Director of the Cardiac Electrophysiology and Arrhythmia Service at Stanford University Medical Center. Dr. Sung graduated from National Taiwan University in 1968. He completed his medical residency at the University of Wisconsin from 1970 to 1972, followed by a cardiology fellowship at the University of Miami from 1972 to 1974. Since then, he has dedicated his research to clinical cardiac electrophysiology. Notably, in 1981, Dr. Sung defined the distinct anatomical basis for human atrioventricular (AV) node dual pathway physiology, which paved the way for catheter ablation as a cure for AV nodal reentrant tachycardia. He later characterized the three forms of ventricular tachycardia—reentry, enhanced automaticity, and triggered activity- relative to therapeutic regimens in 1983 and 1988. Dr. Sung earned a full professorship in medicine at UCSF in 1985 and held that position until 1991. Between 2001 and 2007, he returned to Taiwan, where he served as Dean of the College of Medicine and Vice President of National Cheng Kung University. Over the past 40 years, he has authored and co-authored over 200 original articles and 66 invited chapters and monographs on the mechanisms and treatment of cardiac arrhythmias. His work culminated in the book “Fundamental Approaches to the Management of Cardiac Arrhythmias,” published in 2000.
I dedicate this memoir to my mentor, Professor Agustin Castellanos, Jr., who guided me in Clinical Cardiac Electrophysiology at the University of Miami Miller School of Medicine, to all the “Godsent Angels” who helped me throughout my career as described in this book, and finally to my parents, Mr. and Mrs. Seng-Turng Sung (宋森滕and林冰沁), and my wife, Kuei-Jung Tseng (曾貴榮), to whom I owe a great deal.
Preface
“A journey of a thousand miles begins with a single step.”- Lao Tzu.
For many years, friends have encouraged me to write about my life story. I hesitated for a long time, uncertain of its significance. Then, one day, I realized that my journey from a Taiwanese medical school graduate to a faculty member in the United States could inspire others, especially those aspiring to be medical students and young physicians.
My lifelong aspiration is to be a highly knowledgeable and skilled clinical physician. In 1969, I completed my mandatory service in the Taiwanese Marine Corps, and I embarked on a bold journey with my wife, Kuei-Jung (貴榮), immigrating to the United States.
The 1970s in the United States were turbulent times, marked by the Civil Rights Movement, the Vietnam War, and significant social changes. As a new immigrant, I struggled to establish myself in the medical field while facing language barriers, cultural differences, and the complexities of the American healthcare system. My journey began at a small hospital in Grosse Pointe, Michigan, Bon Secours. With the support of Dr. Richard C. Connelly, Director of Medical Education at Bon Secours Hospital, and Mrs. Madeline McGrath, my landlady, I persevered.
A serendipitous acquittance with Professor Milton Miller (Chairman, Department of Psychiatry, University of Wisconsin-Madison [UWM]) directed me to UWM in July 1970. There, under the mentorship of Professor Robert F. Schilling (Chairman, Department of Medicine), I received rigorous medical training and began to appreciate the importance of research in advancing medical knowledge. More importantly, I met Professor George Magnin of the Marshfield Clinic, who introduced me to the concept of holistic care, a philosophy that has guided my clinical practice ever since.
In 1974, after two years of the cardiology fellowship at the University of Miami, I joined the faculty working under the guidance of Professor Agustin Castellanos, Jr. Together, we pioneered clinical cardiac electrophysiology, exploring the mechanisms of heart arrhythmias and developing innovative treatment strategies. My contributions in this field, including the discovery of the anatomical basis of dual AV nodal pathway conduction, have had a profound impact on patient care. For example, as a result, catheter ablation of slow pathway conduction has become the cornerstone of curing recurrent AV nodal reentrant tachycardia.
In 1981, my academic career continued at the University of California, San Francisco. I collaborated with renowned cardiologists such as Professors William W. Parmley, Kanu Chatterjee, Elliot Rappaport, and Melvin Cheitlin. I focused my research on ventricular arrhythmias and sudden cardiac death, delving into the underlying electrophysiological mechanisms relating to therapeutic consideration for reentry, enhanced automaticity, and triggered activity.
In 1991, I accepted an invitation from Stanford University to establish the section of Cardiac Electrophysiology and Arrhythmia Service. There, I had the privilege of collaborating with world-class clinicians and researchers, including Professors Norman Shumway, Bruce Reitz, Victor Dzau, and Richard Popp, and continued to advance the field through clinical practice, teaching, and research, the latter of which included animal experiment, cellular electrophysiology, AICD (automatic implantable cardioverter/defibrillator) implantation and catheter ablation of various cardiac arrhythmias.
After a decade at Stanford, I returned to Taiwan in 2000 to serve as medical CEO of the Cathay Conglomerate, followed by University Vice-president and Dean of the College of Medicine at National Cheng Kung University (2001-2007) and Distinguished Professor at National Central University. During that time, I shared my knowledge and experience with biomedical colleagues and graduate students, contributing to the development of biomedical research in Taiwan.
In the five years leading up to my retirement in 2012, I shifted my focus to computational cardiac cell modeling of electrophysiology, exploring the underlying mechanisms of inherited ion channelopathies. This research deepened my understanding of arrhythmias and provided valuable insights for new therapeutic strategies.
My journey was a long and winding road filled with challenges and setbacks. Along the way, I integrated clinical practice and academic research, and I received unwavering support from my mentors, friends (godsent angels), and family. Moreover, I had always believed in my faith and resilience. My story will inspire others to persevere in the face of adversity and pursue their dreams.
“Life is a series of unexpected experiences. Persistence in pursuing your dreams can lead you to encounter incredible people (Godsend Angels) who offer support and guidance. These encounters can feel like fate, leaving you to wonder if there’s a hidden pattern or simply the kindness of strangers sent by someone shaping your journey.”- Unknown author.
“Life is uncharted territory. It reveals its story one moment at a time” – Leo Buscaglia (Dr. Love).
Herein, I would like to thank Professor Lien-I Hor (何漣漪), former associate Dean and Professor of Microbiology and Immunology at National Cheng-Kung University Medical College, for editing the Chinese version of this memoir.
To embark on
When I entered the National Taiwan University Medical College (NTUMC) in 1961, I aspired to become a skilled and compassionate physician. The seven-year program consisted of two years of general education, two years of basic science, and clinical training. This clinical training included two years of clerkships and an internship, culminating in a Bachelor of Medicine degree, similar to the British and German systems.
During my clerkship years, John Chaio-An Chang(張兆安), a devout Christian who was also one of my classmates, took me to the “Church Assembly (教會聚會所) “near the medical college. I became more aware of the teachings of Jesus and his disciples as described in the Bible. I remember eagerly gathering every morning around 5:30 am to pray and study the Bible under the guidance of the church elders, Mr. and Mrs. Chi-Chuen Young (楊基銓 劉秀華), whom we fondly called “Papa and Mama Yang.” Together, they introduced me to the teachings of Christ as “loving and forgiving.” These religious beliefs resonated with me, especially given the social and political realities of Martial Law-era Taiwan. As I witnessed the turmoil of society and the suffering of the people, I found solace in messages of hope. In May 1966, I formally embraced Christianity by undergoing a baptismal ceremony. It was a significant turning point in my life, marking the beginning of a more meaningful personal journey.
Jesus answered, “I am the way, the truth, and the life. No one comes to the Father except through me. – John 14:6
“Come to me, all who are weary and burdened, and I will give you rest. Take my yoke upon you and learn from me, for I am gentle and humble in heart, and you will find rest for your souls. – Matthew 11:28-29, NIV.
My Internship Really Solidified My Passion for Medicine.
Interns worked under the supervision of residents and gained hands-on experience working with patients. At that time, interns were required to draw blood and perform microscopic analyses of blood, urine, and stool samples. During my pediatric rotation, I encountered challenging cases. For example, a 16-year-old Taiwanese aboriginal boy developed mental obtundation. Because he had papilledema in the fundus of his eyes, indicating increased intracranial pressure, I suspected he had a brain tumor. However, the brain scan came back normal, and I was puzzled, so I decided to do a lumbar puncture. Since this procedure carried the risk of brain herniation, we prepared for the operation with caution, alert to the possibility of having to inject normal saline for salvation. Afterward, a microscopic examination of the spinal fluid revealed many abnormal round cells that were different from red and white blood cells. Suddenly, I remembered that the boy was a farm worker in a mountainous area, and I suspected that he might be infected with cryptococci. A quick test with Indian ink borrowed from the lab revealed the unique encapsulation of the yeast cells, confirming my suspicion. The next day, I presented my findings on cryptococcal meningitis to the pediatrics department, and they were surprised. Fortunately, the boy received amphotericin B, an appropriate antifungal drug. During his treatment, I became aware of the potential side effects of amphotericin B, in particular, its nephrotoxicity causing acute renal injury, hypokalemia, hypomagnesemia, etc.
In another memorable case, a 36-year-old woman was admitted to the hospital with symptoms of “acute hepatitis.” This case taught me a valuable lesson about the many ways each disease could manifest itself. Despite the initial impression of “acute hepatitis,” her staring eyes and shaking hands led me to request a thyroid function test for hyperthyroidism. While viral hepatitis was a possibility, tests for the virus came back negative, revealing a surprising culprit: hyperthyroidism with thyroid storm. No wonder some people say, “Patients are doctors’ best teachers.”
This case highlighted the far-reaching effects of hyperthyroidism. It could affect numerous organ systems, including the nervous, cardiovascular, gastrointestinal, and skin systems. In some cases, it could even lead to heart failure, with or without atrial fibrillation, and potentially cause long-term damage to the heart muscle (cardiomyopathy). Furthermore, in this high cardiac output state caused by hyperthyroidism, a mismatch between the capacity of the pulmonary and systemic circulations could lead to right-sided heart failure dominating the clinical picture.
My internship was broader than pediatrics and internal medicine. Rotations in surgery, obstetrics and gynecology, and otolaryngology exposed me to a variety of medical challenges. Through it all, however, I became fascinated with electrocardiograms (ECGs). Their ease of use and ability to diagnose heart conditions such as myocardial ischemia and infarction, as well as related issues such as electrolyte imbalances, captivated me. I was particularly drawn to how ECG changes could pinpoint the location of these events (anterior, anteroseptal, etc.) based on coronary artery territories. At that point, I focused on recognizing ECG patterns based on established criteria. In the future, I would have to delve into vector cardiography (ECG in 3-dimensional view) and the underlying cellular changes.
The culmination of my internship was gratifying. It was a great honor to be named “Intern of the Year” by the College of Medicine during Intern Night. This recognition fueled my passion for internal medicine and ignited a spark in me- a desire to specialize in cardiology.
The Decision to Go Abroad
As an intern, I regularly attended lectures and the weekly Clinical-Pathological Conferences (CPC) held at the Seventh Lecture Hall of the National Taiwan University Hospital. These events covered a wide range of medical fields, including internal medicine, surgery, obstetrics and gynecology, pediatrics, etc., and provided me with valuable learning opportunities. I especially enjoyed the sessions with international speakers. They often brought more global perspectives and more accessible explanations, especially on complex topics such as electrolyte imbalances, neurological diseases, and epidemiology. For example, a nephrologist from the United States shared his unique insights into hyponatremia, which gave me a better understanding of the condition. In addition, some young teachers who had been trained abroad often spoke at conferences. Their lectures were rich and inspiring. They not only shared the latest medical research but also told many clinical cases based on their own experiences, which benefited me a lot.
These experiences solidified my desire to pursue postgraduate training overseas, especially in the United States. Studying in an international environment would allow me to gain a broader perspective and more profound knowledge. I could then apply these new insights to future medical practice, providing better treatment for patients.
Dr. Ker-Ming Shan (單克敏): A Pioneer in NTUMC Pediatric Cardiology
Among the faculty, I was particularly impressed by Dr. Ker-Ming Shan, an instructor in pediatrics. Dr. Shan was also a graduate of NTUMC, probably from the class of 1961, and later completed a three-year fellowship in cardiology at the University of Miami. Upon her return to NTUMC, she recognized the need to refine cardiac catheterization techniques. With great initiative, Dr. Shan began training fellow pediatric and internal medicine faculty in this critical skill. At the bedside, she patiently taught interns and residents the art of cardiac auscultation. In the CPC, Dr. Shan analyzed hemodynamic data obtained during cardiac catheterization. She used the data to accurately diagnose various congenital heart diseases, which impressed Professor Shu Yeh (葉𥌓), Chairman of the Department of Pathology. No wonder Dr. Shan won the respect and admiration of most students and doctors alike. When I met her and expressed my interest in studying cardiology, she encouraged me to go abroad.
Dr. Shan was an instructor at NTUMC for eleven years. While the reason why she was passed over for promotion remains unclear, factors such as gender bias, race (because she was not Taiwanese), or personality differences with her superior teachers may have played a role. It is worth noting that during this period, Taiwanese academia retained some aspects of the Japanese hierarchical structure. The traditional apprenticeship model and male chauvinism prevailed in the medical field. Years later, I learned that Dr. Shan returned to the United States and worked at Holy Cross Hospital in Baltimore. Sadly, I also heard that she went through a period of depression and eventually passed away. Recently, while reviewing the official history of NTUMC, I was surprised to find no mention of Dr. Shan’s contributions. Her efforts to establish cardiac catheterization teams in the Departments of Pediatrics and Internal Medicine marked an essential milestone for cardiology advancement at NTUMC. The omission raises questions about the integrity of the historical record, even in contemporary times.
Escape from the Labyrinth: Leaving Taiwan
Leaving Taiwan in 1969 during martial law was like walking through a maze. The government’s overseas policy presented a double dilemma. The Ministry of the Interior required medical graduates like me to serve for two years. At the same time, the Ministry of Education allowed non-medical graduates to emigrate immediately, but they had to be separated from their wives for a year and a half. Determined to leave together, my wife, Kuei, and I devised a strategic plan.
First, I passed the TOEFL and the Study Abroad Qualifying Exam, and then I obtained an I-20 admission form from a U.S. university. Based on these documents, the Department of Education issued me a passport. Meanwhile, Kuei obtained a visitor’s passport through the Overseas Chinese Affairs Committee of the Ministry of the Interior, citing a visit to her cousin, Dr. Kuo-Hwa Tseng (曾國華). I then applied to a U.S. hospital to work as a rotating intern.
When the work contract arrived, I surrendered the I-20, and we applied as a couple at the U.S. Embassy for J1 and J2 visas – the visa combination intended for foreign immigrant workers and their spouses. It was a success. On August 28, 1969, we boarded our first international flight from Taipei to Detroit, Michigan, a trip we will never forget. As the plane took off, a bittersweet realization settled over me. The labyrinthine process we had navigated was a stark reminder of the constraints of martial law. Yet amidst the relief, a wave of conflicting emotions washed over me. Excitement for a new life in the U.S. battled with the deep sadness of leaving our home and beloved parents behind.
Bon Secours Hospital- Rotating Internship (September 1, 1969 - June 30, 1970)
Detroit Served as Our Gateway to the United States.
Upon our arrival, we were greeted by Professor Chun-Ju Lin (林春如) of the Wayne State University School of Engineering. He kindly picked us up from the airport and drove us to Grosse Pointe. It was a quiet residential area with beautiful scenery on Lake St. Clair, in stark contrast to the bustling city. The city was known for auto magnate Henry Ford and his medical center. Dr. Richard C. Connelly, Director of Medical Education, welcomed us at Bon Secours Hospital. Our new home was a room in Mrs. Madeline McGrath’s house in Grosse Pointe, just a few blocks from the hospital. We were very grateful to Kuei’s cousin, Dr. Kuo-Hwa Tseng (曾國華), and his wife, Mrs. Carol Yangzi Su (蘇洋子), for arranging everything before our arrival. Kuo-Hwa was in Psychiatry Residency training at the University of Wisconsin at the time, and Professor Chun-Ju Lin was Kuo-Hwa’s high school classmate.

Visiting Ms. Connelly (second from left) with Mrs. McGrath (first from right) and another intern Aguila from the Philippines, 1969.
The Bon Secours Hospital
This small 150-bed Catholic hospital became my first place of employment in the United States. “Bon Secours” means “Good Help” in French, i.e., “Good Help to Those in Need “. Bon Secours Hospital had over 50 practicing physicians in various specialties and trained approximately 13 interns and residents each year in conjunction with Wayne State University. The hospital’s rotating internship program was a cornerstone of my development. It provided an opportunity to learn not only medicine but also English and the nuances of American healthcare; it was an exciting experience. As an intern, I rotated through five departments – internal medicine, surgery, pediatrics, obstetrics-gynecology, and emergency medicine. Daily interactions with patients and staff honed my medical skills and helped me master the proper pronunciation of medical terms, eventually replacing the Japanese accent I learned in Taiwan.

Residents and Interns (The first very right of the front row: John Muir, the chief resident. Ruey Sung standing at the third from the left of the back row).
The knowledge and medical skills I learned in Taiwan proved to be very useful; Dr. Richard C. Connelly and many practicing physicians quickly recognized my expertise. In a clinical pathology conference (CPC), I correctly identified amniotic fluid embolism as the cause of death for a young woman after childbirth, and in another patient, through analysis of hemodynamic data, accurately diagnosed pulmonary venous thrombosis, which greatly surprised them. In the emergency department, many physicians (mostly non-cardiologists) frequently consulted me on how to interpret their patients’ ECGs. I confidently helped them identify potential cases of myocardial ischemia, infarction, arrhythmias, electrolyte imbalances, and more. As a result, my reputation grew, and I was nicknamed “Rui.” Finally, my paper on the development of artificial pacemakers won first prize and a cash prize of $150.
In September 1969, I began applying for medical residency programs in the United States. Unfortunately, the first 19 university-affiliated hospitals I applied to rejected me. However, persistence eventually paid off; two private hospitals in Detroit, Harper and William Beaumont, accepted my applications. The history of a hospital was an essential consideration in my decision-making process. Harper Hospital was a top medical center in the 1930s, which made it quite appealing to me. In retrospect, language and cultural barriers, as well as the lack of advanced degrees from the country, were challenges I faced in starting an academic medical career in the United States. Despite encountering setbacks, I remained undeterred, believing that God would make the best arrangements. I was comforted by the fact that Harper Hospital’s residency program, like Bon Secours, collaborated with Wayne State University. The decision prepared me for the next chapter of my medical journey.
A Memorable New Year’s Visit to Madison, Wisconsin
In January 1970, I visited my cousin Kuo-Hwa (國華) and his wife, Carol (洋子), in Madison, Wisconsin, for the New Year. The beautiful university town, surrounded by snow-covered lakes and hills, was an unforgettable sight, especially during the holiday season. It was my first time witnessing the spectacle of ice fishing on a frozen lake! The warmth wasn’t just from the scenery – we stayed in their apartment and met their adorable daughters, Fanny and Winin.
However, the highlight of the visit was attending a dinner hosted by the Department of Psychiatry. The occasion was where fate took a surprising turn. At the dinner, I unexpectedly met Professor Milton Miller (1932-2023). The professor, who had become the department chair since the young age of 33, even greeted me in Chinese and said, “How are you!” After understanding my situation, he expressed empathy in a mix of Chinese and English and offered valuable advice.
More importantly, he also arranged for me to meet with Dr. Robert F. Schilling (1919-2014), the renowned Chair of the Department of Medicine at the University of Wisconsin Medical School. The name reminded me of the Schilling test I had learned about in Taiwan. It was used to test for vitamin B12 deficiency diseases such as pernicious anemia. Meeting these two crucial figures – Professor Milton Miller (a genius who received his M.D. at the age of 23) and Dr. Schilling (an academic medical leader known for his research on vitamin B12 and hereditary spherocytosis) – was an honor and, as it turned out, a turning point in my academic medical career. Little did I know at the time that this visit had the potential to reshape my future!
Driven by a desire to pursue a career in academic clinical medicine, I visited the affable Professor Robert F. Schilling. Although he was cautious about my recent arrival in the United States (just four months), he suggested that I apply for an internal medicine residency under him. I immediately wrote to my former professors at the National Taiwan University Medical School, Professor Ming-Tsong Peng (彭明聰), Chairman of the Department of Physiology, and Professor Chiung-Lin Chen (陳烱霖), Chairman of the Department of Pediatrics, asking them to write letters of recommendation for me. I specifically highlighted my accomplishments during my internship at National Taiwan University Hospital – identifying a unique case of cryptococcal meningitis and winning the “Intern of the Year” award.
Despite my optimism, I had a touch of doubt. Upon returning to Bon Secours Hospital, I informed Dr. Richard C. Connelly of the situation. He was very impressed with my potential in internal medicine and cardiology and enthusiastically wrote a lengthy three-page letter of solid recommendation. Dr. Richard C. Connelly’s letter, along with the two letters from Taiwan, significantly boosted my confidence.
A Daring Step and a Life-Changing Decision
After three agonizing months of waiting, I still had not heard from Professor Robert F. Schilling. In late March 1970, John Muir, the chief resident at Bon Secours, encouraged me to take a bold step – he suggested that I call Professor Schilling to inquire about the situation. Despite my trepidation and doubts, I made the call. To my great delight, Professor Schilling informed me that there would be an opening in July due to a resident being drafted into the Vietnam War. He offered me the position, which I eagerly accepted without hesitation.
John Muir was like an older brother to me. He continued to provide unwavering support, helping me write a letter of thanks to Professor Schilling and another letter of apology to Harper Hospital. While accepting Professor Schilling’s position meant an exciting academic future, notifying Harper Hospital also meant breaking my original contract, which brought with it a sense of guilt. I never expected that the phone call I made, inspired by John Muir’s guidance, would be the catalyst for a dramatic shift in my career path from private practice to academia.
The Irony of Fate and the Quest for Peace
I have always been an opponent of war and a staunch advocate for peace. Ironically, my opportunity to pursue residency training at the University of Wisconsin, a crucial step in my career, arose out of the Vietnam War. The internal conflict I felt was a stark reminder of the complexity of life and the often unpredictable twists of fate. Despite my convictions, I recognized the significance of this opportunity and embraced it with determination and gratitude.
This pivotal moment in my life underscored the importance of taking calculated risks, seeking guidance from mentors, and seizing opportunities that align with our aspirations. While I remained committed to my values of peace and nonviolence, I also recognized the need to adapt and navigate the realities of the world around me. This experience gave me a deep appreciation for the power of resilience, adaptability, and the pursuit of excellence in the face of challenges and contradictions.
I bought my first car in preparation for moving to Wisconsin. Gary Venet, an 18-year-old station clerk at Bon Secours, graciously taught me how to drive and even volunteered to drive us to Flint, Michigan. From there, we took a ferry across Lake Michigan to Milwaukee, Wisconsin, and then arrived in Madison. Gary stayed with us for a few days, and I began my medical residency at the University of Wisconsin on July 1, 1970. Gary later went to Los Angeles and strived to become an anesthetist. He also visited us when we were in Miami in 1972. We then lost touch for about 40 years until he contacted us in the summer of 2022. He is now retired and living happily in Hawaii.

Left: Gary and Kuei’s friends visited us in Miami, Florida, in 1972.

Right: Gary and I at Filoli Estates & Garden, Woodside, CA, in 2024.
University of Wisconsin- Medical Residency (July 1, 1970 - June 30, 1972)
Madison
Our new home was in Madison, Wisconsin, the capital city named after the fourth President of the United States, James Madison. This beautiful city, nicknamed “The City of Four Lakes,” sits on an isthmus surrounded by breathtaking bodies of water. It’s also home to the University of Wisconsin (UW)-Madison, known for its pioneering research on warfarin, a blood-thinning medication. Madison’s climate was a welcome relief from Taiwan’s sweltering summers. We settled comfortably into University Town House, a convenient location with a 10-minute drive to the University Hospital.
Diverse training environment
Residency training at UW proved to be more challenging than my internship at Bon Secours. I was the only Asian among 42 residents and interns. To improve my English proficiency, I prepared note cards and rehearsed each one thoroughly to ensure clear case presentations. The residency program worked in teams of two. Each team consisted of a junior (first year) or senior (second year) resident and an intern. We typically admitted two to three new patients each day, resulting in a caseload of approximately twelve patients per team. Our mornings began at 7:30 am with rounds to see all assigned patients, followed by a teaching round led by an attending at around 8:30 am. Throughout the day, we collaborated with a radiologist during a dedicated radiology round to review X-rays and other imaging studies for our patients. Before the end of our shift, we reviewed new laboratory test results and ordered additional tests or consultations with specialists as needed.

Medical Residency II (fifth from left in the front row: Professor Robert F. Schilling. Ruey Sung standing in the 2nd row, 2nd from the right, the only Asian).
In addition to University Hospital, resident physicians here also had to rotate at Veterans Administration Hospital and Marshfield Clinic. Besides general wards, we also gained valuable experience in the ICU and Coronary Care Unit. Our program fostered a solid academic foundation through literature reviews and presentations. As residents at a tertiary medical center, we encountered many complex and uncommon diseases. These included carcinoid syndrome, rheumatoid arthritis, bacterial endocarditis (infection of the heart), pheochromocytoma, primary aldosteronism (adrenal gland tumors), sarcoidosis, hyperparathyroidism (parathyroid hyperactivity), multiple sclerosis, scleroderma (connective tissue disease), chronic ulcerative colitis (inflammatory bowel disease), syndrome of inappropriate antidiuretic hormone secretion (SIADH), and more.
“Code blue” scenarios, meaning in-hospital cardiac arrest, required immediate action. Recognizing the critical role of the on-call resident in leading resuscitation efforts, I took the initiative to hone my skills further. I secured a month-long rotation in anesthesiology. Early mornings at 5:00 am were dedicated to mastering endotracheal intubation, a technique critical to ensuring an airway in surgical patients. This rotation also equipped me to insert an IV line into the subclavian vein, another vital tool in critical care situations. These techniques proved invaluable in “code blue” emergencies and in managing patients in the Intensive and Coronary Care Units.
Our coursework encompassed literature reviews and presentations, supplemented by foundational textbooks like Cecil’s and Harrison’s. To broaden my medical knowledge, I actively explored medical journals such as the Annals of Internal Medicine and the Journal of the American Medical Association. This comprehensive approach proved invaluable. I firmly understood and memorized essential knowledge while managing patients, such as the oxyhemoglobin dissociation curve, the steady-state relationship between the serum creatinine concentration (Cr) and the kidney glomerular filtration rate (GFR), etc. I recalled impressing my attending physician by identifying the potential link between carcinoid syndrome and right-sided valvular heart disease (i.e., pulmonic stenosis, tricuspid regurgitation), as well as the atypical presentation of hyperthyroidism (e.g., apathetic hyperthyroidism) in elderly patients. Through diligent self-study and diverse clinical experiences, I made substantial strides during my residency training.
The fast pace of the residency kept me on my toes. One day, a woman in her 70s in a semi-comatose state arrived in the emergency room. Her smoking history, physical signs of emphysema, decreased respiratory movement, and swollen optic nerve head (papilledema) led me to suspect “CO2 narcosis” as the cause. Indeed, later on, an arterial blood gas test confirmed my suspicion and revealed a dangerously high CO2 level (over 95 mmHg, compared to the standard of 40 mmHg) before I intubated her. Carbon dioxide vasodilates the brain and results in brain swelling (intracranial pressure elevation and papilledema) due to increased blood flow. This case and countless others exemplified the rigorous and immersive learning environment of the residency program. These invaluable experiences laid a solid foundation for my future academic research and clinical career.
Dr. Sung-Feng Wen (溫松峰) – A Model Scholar
In contrast to Taiwan’s hierarchical medical education system, it was common here to call attending physicians by their first names. I was particularly impressed by a dinner party where the former Chairman of the Department of Medicine, Dr. Ovid O. Myer, personally came to meet us and kindly invited Kuei to dance. On campus, we also met some Taiwanese graduate students. On a personal level, we met Dr. Wen Sung-Feng (溫松峰) (NTUMC Class of 1958), an assistant professor of internal medicine who specialized in using micro-puncture techniques to study kidney function. Professor Wen’s humility, courtesy, and demeanor left a lasting impression on us. He and his wife once invited us their home for a delicious dinner. Dr. Wen’s demeanor deeply impressed me as a model for Taiwanese medical professionals, especially professors at NTUMC, my alma mater. His achievements in the field of medicine strengthened my confidence in pursuing a clinical academic career in the United States.
Marshfield Clinic – Holistic Medicine Training
In September 1970, I rotated to the Marshfield Clinic, about 100 miles to the north. There, I met Dr. George E. Magnin (1922-2019), a clinical professor of internal medicine and a general internist. He believed that internists should be trained as generalists before specializing. Dr. George Magnin had a passion for teaching and an unstoppable enthusiasm for teaching medical students and residents.
One night, which I will never forget, he woke me up at 2:00 in the morning to examine a patient with chest pain. Based on the ECG results and elevated cardiac enzymes, we jointly diagnosed an acute myocardial infarction. Dr. Magnin carefully reviewed the key physical examination findings and laboratory results. Then he asked me a question: “Rui, is this patient’s low-grade fever and leukocytosis related to acute myocardial infarction, or could it indicate some other problem, such as a bacterial or viral infection?” The question led me to think deeply about whether the patient had problems in other organ systems, such as the urinary, gastrointestinal, or endocrine systems.
Dr. George Magnin also required me to study some recent literature and then discussed in rounds some potential scenarios, such as how to assess the severity of heart failure in chronic obstructive pulmonary disease (COPD), the latest treatment for cardiogenic shock, and so on. This was valuable training in holistic medicine.
The Marshfield Clinic philosophy emphasized patient-centered care. More uniquely, physician salaries were based on seniority rather than specialty, encouraging close collaboration among physicians in holistic medicine. Marshfield Clinic was known as “The Mayo Clinic of Wisconsin” because of its reputation for medical excellence. All residents who had trained at Marshfield Clinic valued the teachings of Dr. George Magnin. His dedication to education had earned him the well-deserved title of “The Internist of the State of Wisconsin”-(https://www.facebook.com/marshfieldclinic/videos/dr-george-magnin-marshfield-clinics-great-teacher/2374799395940295/).
Two Other Unforgettable Mentors
At the University Hospital, an associate professor, Dr. Neville Bittar, significantly influenced my approach to patient care in the Coronary Care Unit. He posed a thought-provoking question: “Is isoproterenol infusion ever an appropriate treatment for acute myocardial infarction? This quiz prompted me to delve into the pharmacology of various pressor agents and their contrasting effects on ischemic cardiac tissue. Dr. Bittar further enriched my clinical skills by meticulously teaching cardiac auscultation. He sharpened my ability to identify mitral valve prolapse through characteristic sounds such as the “mid-systolic click” and the “late systolic murmur” influenced by changes in posture. Drs. Magnin and Bittar taught me the invaluable skills of critical analysis during medical evaluations, lessons that continued to shape my practice.
During my rotation at the Veterans Administration Hospital, I met Dr. Kelvin M. Kunin, Vice Chairman of the Department of Medicine. He was a renowned infectious disease specialist known for his expertise in antibiotic resistance. In particular, he taught us how to examine infected material, whether it was sputum collected or pus and effusion drained – by appearance, color, and odor. I remembered him mentioning during a case discussion that we should always use the generic name for a drug, not the brand name. One day, accompanied by Kuei, I visited him in his office to introduce ourselves and share more about our motherland, Taiwan. At that time, people often confused Taiwan with Thailand. He was pleased to see us. I couldn’t imagine that some thirty-six years later, he would be a visiting professor at the National Cheng Kung University College of Medicine, where I served as Dean.

Medical Residency II (fifth from left in the front row: Professor Kelvin Kunin. Ruey Sung standing in the back row, third from the right).
The Five-Finger Approach: A Cornerstone of Clinical Assessment
In the 1970s, Dr. Proctor Harvey at Georgetown University introduced the ‘five-finger approach’ for diagnosing cardiovascular disease. This method, still widely used today, relies on a comprehensive patient history, physical examination, ECG, chest x-ray, and basic laboratory tests (Ref. 1).
The patient history is crucial, often providing the key to diagnosis in 75-80% of cases. It covers five essential areas: chief complaint, present illness, past medical history including current medications, personal/social/family history, and review of systems. The physical exam focuses on five key signs: body habitus, venous and arterial pulsations, chest movement, and heart sounds. A resident physician must be proficient in interpreting acid-base balance (via arterial blood gases), estimating central venous pressure, identifying cannon A waves (via neck jugular veins), and recognizing atrial fibrillation, pulsus paradoxicus, and pulsus alternans (via radial pulse and sphygmomanometer). A skilled physician, like a detective, uses these clues garnered from the five-finger approach to identify the underlying cause of the patient’s illness.
While additional tests may be needed for confirmation, the physician’s role is to tailor the treatment plan to each patient’s unique circumstances, considering risk factors and comorbidities. Effective communication with patients and their families is essential for providing optimal care. Patients also have the right to seek consultations or second opinions. These principles of patient-centered, holistic care have guided my clinical practice in academic medicine.
Through diligent participation in rounds and patient care, I relentlessly pursued knowledge and experience in internal medicine. As I neared the end of my first year of residency, Professor Robert Schilling, Chairman of the Department of Medicine, invited me into his office. My initial apprehension quickly vanished when I learned that he was offering me a second-year residency position based on the favorable recommendation of Professor George Magnin. He remarked happily, “Professor George Magnin is of the opinion that you are more diligent than our American residents and strongly recommends that we retain you.” His words deeply moved me.
Throughout his career, Professor Robert Schilling emphasized the importance of humility, often quoting phrases such as “being in the right place at the right time” and “there is no such thing as a free lunch.” These words resonated with me and made me wonder if Professor Schilling’s decision to accept me as a first-year and retain me as a second-year reflected his expectations of my potential beyond my efforts. Regardless, I was immensely grateful for the support and encouragement I received from Professors Milton Miller, Robert Schilling, George Magnin, and Neville Bittar at the University of Wisconsin, as well as Dr. John Muir (the Chief Resident) and Director Richard Connelly (the Director) at Bon Secours Hospital.

1985, Kuei and I hosted a luncheon for Dr. and Mrs. George Magnin while they were visiting San Francisco. They were glad to hear that I had already been promoted to Professor of Medicine at the University of California at San Francisco (UCSF).

Family Support
In 1971, my wife’s parents arrived from Taiwan and provided invaluable support during a demanding time. Their help was invaluable as I prepared for my internal medicine board exam and applied for cardiology fellowship training. During our free time, I took them on trips around Madison and the surrounding area, even venturing to the Wisconsin Dells and the Milwaukee Zoo in the spring. This was also the year that Taiwan gave up its seat at the United Nations, ceding it to Beijing. To explore future options, I accompanied them to Chicago for a consultation with an immigration lawyer. When the United States finally recognized China in 1978, their family situation evolved. In 1980, my wife’s parents and five of their eight children moved to Kobe, Japan.
Kuei’s parents at the University of Wisconsin-Madison Campus, 1971.
From J1 to H Visa
During this time, the Immigration Service announced a new policy due to the Vietnam War. To address the shortage of medical personnel, they allowed J visa holders to convert to H visas. This H visa category designated me as a “specialized alien” needed in the U.S. and eligible to apply for permanent residence within five years. This unforeseen shift in immigration policy offered the possibility of staying in the U.S. long-term, a prospect I hadn’t previously considered. Another unexpected benefit was that the U.S. recognized my military service in Taiwan, including basic training and a year in the Taiwanese Marine Corps. Unlike many young American doctors who hadn’t served, these credits placed me in a low-priority category for the draft.
From Internal Medicine to Cardiovascular Medicine
For my cardiology fellowship, I narrowed my applications to two. Unexpectedly, within a week, Dr. Robert Boucek, Chief of Cardiology at the University of Miami (UM), offered me a fellowship funded by a National Institutes of Health (NIH) grant. UM was my first choice because I greatly admire Professor Agustin Castellanos, Jr. from the school, who could elegantly apply vectorcardiography to explain the characteristics of ECG. Eager to learn more, I self-funded a trip to Miami to meet Drs. Boucek and Castellanos in person. Dr. Boucek struck me as a sincere and genuine leader, while Dr. Castellanos embodied humility and scientific excellence. Although tempted by the prestigious reputation of the Mayo Clinic in Rochester, Minnesota, I ultimately withdrew my application not to wait for the final decision. The Mayo Clinic’s national and international referral base and its affiliation with the Mayo School of Medicine were indeed attractive. However, the opportunity to work with Dr. Castellanos and Dr. Boucek at UM, with the added benefit of immediate acceptance, solidified my decision. After returning to Madison, I immediately notified Professor Robert Boucek of my decision to join them. It was a joyous moment!
Wisconsin winters could be brutal, with temperatures dropping to -10°C. Without a garage in our apartment, car trouble was common. In February 1972, I accidentally overheated my car’s engine. Fortunately, it was under warranty, and the dealership surprisingly replaced the engine free of charge. Knowing that we would soon be moving our family to Miami, Florida, I traded the car in for a more practical station wagon. With myself behind the wheel, we took a four-day road trip south through Illinois, Kentucky, Tennessee, and Georgia to our new home in Miami.
References
(1) Leonard JJ. W. Proctor Harvey. Profiles in Cardiology edited by J. Willis Hurst. Clin Cardiol 1991;14:180-181.
University of Miami (UM)- Cardiology Fellowship (July 1, 1972- June 30, 1974)
The Miami Home- a Pink House
Thanks to my NTUMC alumnus, Dr. Cheng Kai Yu (余正凱), we secured a rental house in Coral Gables, Miami. Located near the main campus of the University of Miami, it would be a 30-mile drive to my fellowship hospital, Jackson Memorial. The charming pink three-bedroom house had a yard full of mango trees. Our landlords, the Kruses, Margaret and Robert, lived nearby and graciously mowed the lawn weekly. We were fortunate to develop friendships with our neighbors, the Grays. Ralph Gray, a retired attorney, and his wife, Julie Gray, often called on me for medical advice, fostering a warm sense of community.
Miami, Florida, was a stark contrast to Grosse Pointe, Michigan, and Madison, Wisconsin. With a population of 2.31 million, Miami was the largest city in the sprawling Miami-Fort Lauderdale-West Palm Beach metropolitan area in the southeastern United States. Nicknamed the “Gateway to Latin America,” Miami thrived on its deep commercial and cultural ties to the region. Spanish was a widely spoken language, which was evident in vibrant neighborhoods such as Little Havana. The city also embraced academia as home to the prestigious University of Miami, a private institution founded in 1925.
Jackson Memorial Hospital
Jackson Memorial Hospital, the primary teaching hospital of the University of Miami, was located in Miami-Dade County. Renowned nationwide, it boasts 1,500 beds, a bustling emergency room, and a state-of-the-art nationally reputed Coronary Care Unit (CCU). Led by Professor Louis Lemberg (1916-2012), this advanced CCU featured eight private rooms. The heart of the unit was a work area designed specifically for doctors and nurses, arranged in a semi-circular layout and surrounded by these rooms. Equipped with portable fluoroscopy and resuscitation equipment, the CCU provides comprehensive care. Each room was furnished with cardiac monitoring systems, pulse oximeters, ventilators, oxygen supply, intravenous lines, respirators, and hemodynamic monitoring systems.
What was particularly special was that the CCU was connected to a large, modern audiovisual classroom that was used to provide CCU training courses for doctors and nurses. Professors Louis Lemberg and Agustin Castellanos, Jr. were dedicated educators, constantly developing CCU courses for doctors and nurses nationwide. They also held annual symposia at Walt Disney World in Orlando, Florida, to teach innovative methods of coronary care. While I was eager to delve deeper into vectorcardiography with Dr. Agustin Castellanos, my primary focus during my cardiology fellowship was still mastering clinical cardiology – an extension of internal medicine. I was fortunate to learn from two associate professors, Dr. Ali R. Ghahramani (1930-2015) and Dr. Stephen M. Mallon, who helped me sharpen my clinical skills and gain expertise in cardiac catheterization techniques.
Sudden Death, Silent Myocardial Ischemia, and Atypical Aortic Dissection
The first genuinely challenging case I encountered in the CCU was a 65-year-old man who experienced sudden death syndrome. One morning, while brushing his teeth, he suddenly collapsed without warning. Paramedics recorded ventricular fibrillation (VF) and successfully defibrillated him. Admitted to the CCU, he lost consciousness again without warning due to VF and required defibrillation once more.
The next day, as I prepared for cardiac catheterization and coronary angiography, I was startled by an ECG showing widespread ST-segment elevation, indicative of an acute anterior wall myocardial infarction. Surprisingly, the patient had no chest pain. I quickly sent him back to the CCU, only to find that the ECG abnormalities had disappeared entirely.
A realization dawned on me: this was a case of asymptomatic (atypical) coronary artery spasm or “Prinzmetal angina.” Subsequent angiography confirmed that, apart from a mild (30%) stenosis in the left anterior descending artery (LAD), the rest of the coronary artery system was normal. Interestingly, during the procedure, ergonovine infusion induced a spasm of the LAD, leading to VF and loss of consciousness. Again, the patient had no chest symptoms prior to the onset of VF. Fortunately, JMH had verapamil, a calcium channel blocker. Verapamil therapy successfully controlled and prevented further recurrence of spasms. This case highlighted the asymptomatic nature of myocardial ischemia (silent myocardial ischemia), which often occurs in diabetic patients, the older, and women.
Later, a 68-year-old businessman from New York was transferred to the CCU due to chest pain radiating to his back and an electrocardiogram (ECG) suggestive of inferior wall myocardial ischemia, likely involving the right coronary artery. He had a history of hypertension, and upon physical examination, I noted cardiomegaly and a grade 2/6 aortic regurgitation murmur, a common finding in chronic hypertension. However, a chest X-ray revealed a widened mediastinum, raising suspicion of acute aortic dissection.
Despite normal blood pressure readings in both arms, I urged for an immediate cardiac catheterization. The procedure confirmed the diagnosis of aortic dissection extending from the aortic root to the bilateral subclavian arteries, with a systolic blood pressure of 200 mmHg in the proximal aorta and left ventricle. Given the severity of the condition (Type A aortic dissection), the cardiovascular surgical team performed an emergency operation, replacing the aortic root with a Dacron graft and performing coronary bypass grafting on the right coronary artery.
These two complex cases sparked my passion for cardiology and fueled my ambition to become a skilled cardiologist.
Prolapsing and Non-Prolapsing Atrial Myxomas
Associate Professor Ali Ghahramani, an Iranian immigrant educated in Britain, was renowned for using “kindness and laughter as an elixir of healing.” His gentle and polite demeanor earned him the nickname “Dr. G” among patients. I learned from him how to become a doctor beloved by patients. One morning, we encountered a case of a 22-year-old female patient complaining of intermittent fainting and shortness of breath. During the examination, Ali Ghahramani and I detected an intermittent systolic murmur at the apex, suggesting mitral regurgitation. At that time, echocardiography technology was still in its early stages, with only M-mode ultrasound available. This technique revealed an atrial myxoma in the left atrium, a rare tumor mass attached to the atrial septum. Clinically, myxomas can mimic symptoms of connective tissue diseases and may lead to the formation of thrombi and tissue debris around the tumor, causing systemic embolism. We quickly arranged a surgical consultation. She underwent tumor resection surgery and recovered successfully.
Inspired by this case, I immediately collected four similar cases previously seen at JMH. Collaborating with echocardiographer Dr. Steve Gottlieb, I reviewed the dynamic relationship between the hemodynamic characteristics and M-mode echocardiography findings of these five cases. We then classified these cases into “Prolapsing” and “Non-Prolapsing” types, providing a reasonable explanation for the intermittent symptoms of mitral stenosis and mitral regurgitation that patients experienced due to positional changes. Specifically, in the non-prolapsing state, the tumor can obstruct pulmonary venous drainage, leading to symptoms of heart failure. Alternatively, if the tumor prolapses into the mitral valve orifice, it can cause dizziness and fainting spells (syncope). In both scenarios, the patient is at risk of embolic stroke. This research became my first published article in the medical literature (Ref. 2). Because it was published in Circulation, a top journal in clinical cardiology, it caught the attention of our cardiology department. Soon after, my second article, co-authored with Associate Professor Dr. Stephen Mallon, was also published in Circulation, focusing on analyzing the role of saphenous vein coronary artery bypass grafting in patients with left main coronary artery obstruction (reference 3).
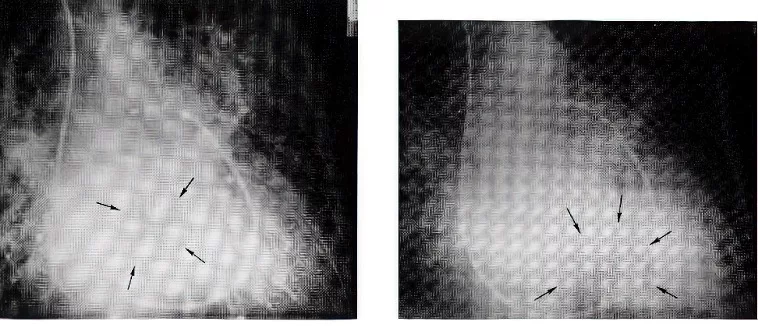
Left two figures: A prolapsing left atrial myoma: Levo phase of pulmonary angiograms in right anterior oblique projection. Photographs have been retouched in order to better delineate the tumor in the left atrium during systole (left) and in the left ventricle during diastole (right) in contrast to the non-prolapsing left atrial myoma, which remains in the left atrium during both systole and diastole.
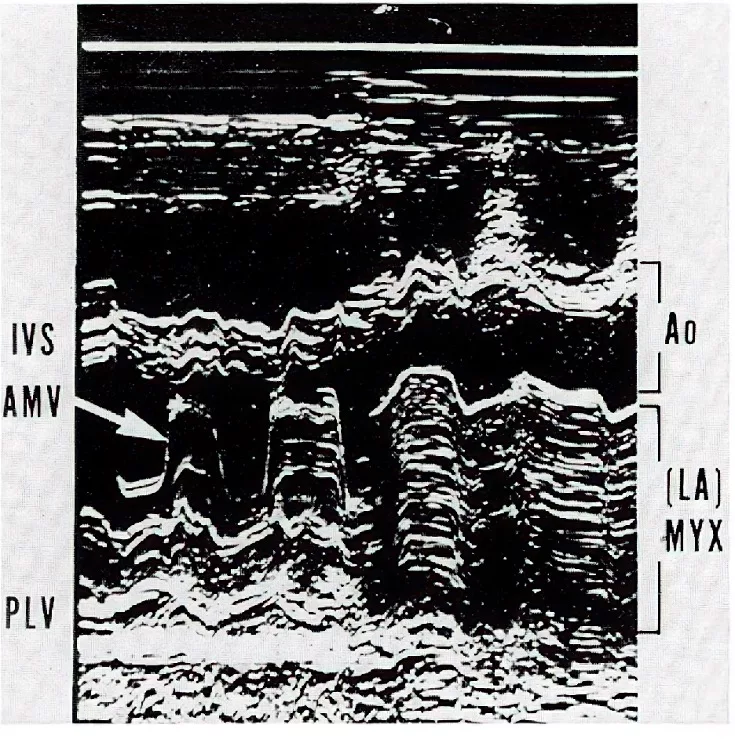
Right figure: The M-mode echocardiogram showing prolapse of a left atrial myxoma entering the left ventricle through the mitral annulus (arrow). IVS: interventricular septum; AMV: anterior mitral valve; PLV: posterior left ventricle; Ao: aorta; LA: left atrium; MYX: myxoma (Reference 2 with permission from Circulation).
Cardiology Fellowship Training Program: A Rich and Diverse Experience
JMH’s location and reputation made it a leader in cardiovascular medicine in the Southeast. In addition to the Department of Cardiology, cardiovascular medicine boasted prominent figures: Dr. Gerald A. Kaiser led cardiovascular surgery with esteemed surgeons such as Hooshang Bolooki and Richard J. Thurer. Pediatric Cardiology was led by Dr. Henry Gelband, with Dorothy Tamer, Otto L. Garcia, and Grace Wolff on staff.
As cardiology fellows, we took part in weekly clinics and clinical service, rotating through the CCU to assist residents under the guidance of the CCU director and the cardiac catheterization laboratory to refine our technical skills. Our responsibilities in the cardiac catheterization laboratory included holding weekly meetings with cardiac surgeons to discuss potential cases for surgical intervention and providing a comprehensive understanding of managing cardiovascular disease in various clinical situations. We frequently collaborated with general medicine and emergency department (ED) residents to ensure timely consultation. Of these rotations, the CCU was particularly rewarding. There, I further developed my skills in acute cardiac care, managing patients with conditions such as myocardial infarction, heart failure, respiratory failure, arrhythmias and electrolyte imbalances, and cardiogenic shock.
The foundation established during my internal medicine residency at the University of Wisconsin proved invaluable in confidently navigating the demands of a cardiology fellowship at JMH. One morning, an ER physician called me to see a 68-year-old woman with a recent syncopal episode preceded by palpitations. She had a history of hypertension and paroxysmal atrial fibrillation (AF). Two weeks prior, a clinic physician started her on digoxin 0.25 mg daily. Her detailed history was essential for diagnosis and treatment. She reported increased palpitations since starting digoxin and had passed out two hours before arriving at the ED. While in normal sinus rhythm, I detected left ventricular hypertrophy with a forceful impulse at the apex with a grade 2/6 systolic murmur at the left lower sternal border. A Valsalva maneuver increased the murmur to grade 4/6. I urgently ordered an echocardiogram (only the M mode was available in 1972), which confirmed the diagnosis of hypertrophic obstructive cardiomyopathy (HOCM). I switched digoxin to a beta-blocker (propranolol) and adjusted her antihypertensive regimen. I explained to the resident that digitalis is contraindicated in HOCM due to its positive inotropic effect, which could worsen left ventricular outflow tract obstruction and promote the genesis of AF, leading to syncope and sudden death. At the one-week follow-up, the patient was feeling much better.
Upon completion of the fellowship, I was honored to receive the Martin H. Stein Memorial Award for “outstanding performance as a cardiology fellow (1973-1974).” This award, a testament to my proactive learning and fulfilling my duty in patient care, also recognized my ability to complete two research projects accepted by the Circulation Journal, which stood out among the rest of the cardiology fellows. The recognition of my efforts with this prestigious award had a profound impact on my career, opening doors and opportunities. Subsequently, I passed the American Board of Internal Medicine examinations in Internal Medicine and Cardiovascular Medicine in 1974 and 1975, respectively.
Professors Agustin Castellanos, Jr. and Sr. – Father and Son
Professor Agustin Castellanos, Jr. (known as “Tino”), who later became my mentor, had a fascinating family background. While Tino’s family had Cuban roots, his appearance did not fit the typical Latin stereotype. Family lore suggested that a Chinese ancestor, possibly surnamed Chen—a common name in Southern China—was brought to Cuba as an enslaved person. The Spanish surname “Castellanos” might have been acquired from their enslaver. Tino’s wife, Maria, was amicable and considerate, adding a touch of elegance to her Hispanic heritage. She was well-liked and respected by residents and fellows.
During my cardiology fellowship, I had the privilege of training for two months with Tino’s father, Professor Agustin Castellanos, Sr. (“Chino”). A renowned pediatric cardiologist and pioneer in pediatric cardiac catheterization, Dr. Castellanos Sr. was also a three-time Nobel Prize finalist. His mentorship greatly enriched my understanding of congenital heart disease. Both father and son were known for their humility and kindness despite their impressive social and academic accomplishments. The affectionate nicknames “Chino” (meaning “Chinese”) for the father and “Tino” (little Chino) for the son added a personal touch to their remarkable legacy.
Professor Castellanos, or “Tino” as he was known, excelled at teaching vectorcardiography using clear and concise visuals. His lectures enabled me to apply vector concepts in three dimensions – frontal, horizontal, and sagittal planes – and guided me in understanding the 12-lead ECG characteristics (six limb and six chest leads) better. I mastered the recognition of key patterns, including those associated with acute myocardial infarction with modifications such as hemiblock (left anterior and left posterior hemiblock), bifascicular block (right bundle branch block with left anterior or left posterior hemiblock), differentiation between left ventricular hypertrophy and left bundle branch block, separating true posterior wall myocardial infarction from right ventricular hypertrophy, diagnosing biventricular hypertrophy, and identifying the ventricular pacing site of the pacemaker and the location of the A-V bypass tract (ventricular preexcitation).
This knowledge was crucial while working in the CCU. The house staff (interns, residents, and nurses) were often impressed by my ability to pinpoint problems such as temporary lead dislodgement – whether in the coronary sinus, right ventricular inflow, or outflow tract. As a result, I was able to adjust the pacing wire position without relying on fluoroscopy. Remarkably, these same principles remain relevant today, as they are used in electrode catheter ablation procedures for localizing A-V bypass tracts and the origins of other arrhythmias, such as ventricular premature beats and ventricular tachycardia.

Professor Agustin (“Tino”) Castellanos, Jr. with his father, Agustin (“Chino”) Castellanos, Sr., and elder son, Danial (right) (Miami, Florida).
Opportunity or Destiny? The Connection to Dr. Ker-Ming Shan (單克敏)
One evening, as I hosted Professor Leonard S. Sommer, director of the cardiac catheterization laboratory, and his wife for dinner, an unexpected name emerged from our conversation. Professor Sommer mentioned that several years ago, a cardiologist from Taiwan named Dr. Ker-Ming Shan(單克敏) completed a three-year fellowship under his tutelage. She was exceptionally dedicated and highly skilled.
I was heartened to hear Professor Sommer’s praise for Dr. Shan. Yet, I couldn’t help but think of the challenges she had faced and the unfair treatment she endured at her alma mater, National Taiwan University College of Medicine. Despite these hardships, Dr. Shan had been a guiding light for me, encouraging me to pursue my academic aspirations in the United States. However, she had never mentioned the University of Miami/Jackson Memorial Hospital (UM-JMH). This serendipitous discovery during dinner felt like a cosmic sign, a hint of destiny. Was it mere coincidence, or was I following a path that had been laid out for me?

Professor and Mrs. Sommer visited Taiwan in 1973, and Ruey’s brothers hosted them in Taipei City.
Another Turning Point in Journey
In June 1974, as I was nearing the completion of my cardiology fellowship, an exciting turn appeared in my future. As the recipient of the Martin H. Stein Memorial Award for Cardiology Fellowship Training, I dreamed of obtaining an academic position at the University of Miami-Jackson Memorial Hospital. Unfortunately, there were no vacancies at the time. While exploring private practice opportunities, I met with two private cardiology practicing groups in Miami and Winter Haven. Both expressed interest. However, due to its proximity to Walt Disney World, I was leaning toward the practicing group in Winter Haven.
Just in August 1974, as I returned from visiting my parents in Taiwan and was preparing for final negotiations with the Winter Haven group, Tino presented me with an opportunity that would change the course of my life. He told me that Robert J. Myerburg, the chief of cardiology, and he were planning to establish a new Clinical Cardiac Electrophysiology department. They had secured sufficient school resources and were recruiting two new faculty members, with me being their top choice. It felt like a déjà vu moment, reminding me of my dramatic acceptance to the University of Wisconsin as a resident physician during my internship at Bon Secours Hospital in 1970. I grabbed this opportunity without hesitation, as it perfectly aligned with my initial desire to continue teaching and research at the University of Miami-Jackson Memorial Hospital with Tino. It was another joyful and grateful day for me!
University of Miami - Cardiac Electrophysiology (July 1, 1974 - February 28, 1981)
In the 1970s, the University of Miami – Jackson Memorial Hospital (JMH) was already known for its state-of-the-art Coronary Care Unit (CCU) and had a team of distinguished basic science cardiac electrophysiology researchers. This group included Robert J. Myerburg, Arthur L. Basset, Henry Gelband, Ralph Lazara, and Benjamin J. Scherlag, all of whom had trained in the microelectrode laboratory of the late Brian F. Hoffman (1925-2013) at Columbia University. Nabil E. El-Sherif later joined them. Of them, Benjamin J. Scherlag pioneered the recording of the human His bundle electrogram (Staten Island Public Health Service Hospital) in 1969 (Ref. 4).
The advent of His bundle recording technique created the field of clinical cardiac electrophysiology. Tino and I at UM were determined to be at the forefront of this new field. The leading programs at that time were the Staten Island Public Health Service Hospital (Anthony N. Damato, Benjamin J. Scherlag [later, joined UM], Masood Akhtar), the University of Illinois (Kenneth Rosen, Pablo Dennis, and Delon Wu), Duke University (John J. Gallagher, Andrew G. Wallace, Eric N. Prystowsky, and Will C. Sealy), The University of Pennsylvania (John Kastor and Mark E. Josephson). There were also a few groups internationally, in the Netherlands, France, and Italy. Dirk Durrer led the Dutch group, which included Heins J. J. Wellens and Reinier M. Schuilenburg as collaborators.
The establishment of a clinical cardiac electrophysiology program at JMH was a pioneering effort. Unlike other institutions, JMH did not have a dedicated electrophysiology (EP) laboratory. As a result, I had to wait for the cardiac catheterization team to finish their coronary angiograms before I could perform EP studies. As a result, my procedures often began well after 4:00 p.m., earning me the nickname “Four O’clock Rui.” Frank (Francisco Garcia-Montes), the senior catheterization laboratory technician, became a close friend and invaluable ally. He kept me informed of the schedule so that I could maximize my efficiency. His friendship meant a lot to me, and his passing years later was a significant loss.
Data Collection and Interpretation
My exploration of clinical cardiac electrophysiology began with a deep dive into the basic science. I immersed myself in the work of prominent investigators such as Gordon Moe, Anton Becker, Robert Anderson, Michiel J. Janse, Maurits A. Allessie, and Paul F. Cranefield, gaining insights from their studies of animal and cellular cardiac electrophysiology. The knowledge garnered by these efforts provided a strong foundation for my work.
My initial study technique followed established protocols for recording electrical activity in the heart. As described by some of the pioneers, I used a fluoroscope to precisely position a tetrapolar catheter near the atrioventricular (AV) junction to capture His bundle signals. Two additional tetrapolar catheters were placed in the high right atrium (HRA) and right ventricular apex (RVA) for both recording and periodic electrical stimulation. To study arrhythmia initiation and termination, I usually performed programmed premature extra stimulation in HRA and RVA at driving cycle lengths of 400 and 600 milliseconds, along with progressive rapid stimulation. As my knowledge expanded, I incorporated pharmacological agents into my studies to further elucidate the mechanisms of cardiac arrhythmias.
In the absence of a tape-recording system in the EP laboratory, Frank employed a laborious printing process for all data rolls. This meticulous approach provided a wealth of data for me to analyze independently at home before consulting with Dr. Tino. These Saturday morning sessions in Tino’s office were a cherished time of collaboration and camaraderie. His generosity often extended to a delightful post-meeting lunch at a Cuban restaurant, where I developed a lasting love for paella, the spicy saffron rice dish.
The Right Bundle Branch Block ECG Pattern
In the context of electrocardiograms (ECG), the origin of the right bundle branch block (RBBB) pattern can be either central or peripheral, which may have different clinical implications. Tino and I first discussed how to use the conduction time between the His bundle and the right ventricular apex (H-RVA interval) to differentiate its origin. We later demonstrated that, despite having complete or incomplete RBBB patterns, patients with atrial septal defects (ASD) had H-RVA intervals similar to those of normal individuals, indicating that their RBBB pattern was “peripheral” rather than “central.” The fundamental principle is that the left-to-right shunt in ASD led to right ventricular volume overload and stretching of the Purkinje fibers, resulting in this peripheral RBBB pattern. We believed this peripheral RBBB pattern was benign and could normalize after ASD closure (reference 5).
Conversely, in cases of Tetralogy of Fallot, the RBBB pattern resulting from surgery was more complex because it involved both right ventriculotomy and ventricular septal defect (VSD) repair (reference 6). While the right ventriculotomy disrupts the Purkinje fibers within the right ventricular muscle, leading to a “peripheral” RBBB pattern, surgical repair of VSD could result in a “central” RBBB due to its proximity to the His bundle area. Theoretically, if the left bundle branch is damaged later in life, the risk of progression from a central RBBB pattern to a complete heart block might ensue.
Nevertheless, we now know that the ECG pattern of “complete RBBB” is not anatomically or functionally definitive. If the right bundle branch completely loses its conduction ability, the QRS complex duration of an RBBB pattern must exceed 160 milliseconds, not the 120 milliseconds defined in conventional electrocardiography. Furthermore, the surgical scar and surrounding tissue in the right ventricle, resulting from the incision (i.e., right ventriculotomy), can serve as a substrate for reentrant circuits, prone to ventricular tachycardia (VT), which can potentially deteriorate into ventricular fibrillation (VF). VT leading to VF is recognized as the primary mechanism for sudden cardiac death in postoperative Tetralogy of Fallot patients.
His Bundle Mapping and Pacing
In my early role, I also worked with Dr. Gerald Kaiser, the Chairman of the Cardiovascular Surgery, to map His bundle activity in the operating room. This procedure was performed on children with congenital heart defects such as ventricular septal defects and endocardial cushions. As an adult cardiologist, I appreciated the opportunity to interact with pediatrics and cardiovascular surgery. Later, Tino encouraged me to explore his bundle pacing. He specifically asked me to recognize various ECG patterns produced from purely total His bundle stimulation to different degrees of partial His bundle stimulation fused with local myocardial activation.
While I had mastered static electrophysiology (ECG pattern recognition), I craved a deeper understanding of dynamic electrophysiology. This field focuses on the mechanisms behind cardiac tachyarrhythmias, particularly atrioventricular (AV) nodal reentrant tachycardia and AV reciprocating tachycardia associated with Wolff-Parkinson-White (WPW) syndrome. I was particularly interested in factors that influenced arrhythmia initiation, termination, and response to various medications. By this time, I had solidified my foundation in the basic principles of cardiac electrophysiology. These included the three main basic mechanisms of arrhythmias (enhanced automaticity, triggered activity, and reentry) and the essential elements for reentrant tachycardia: an appropriate reentrant circuit, a triggering event, and sufficient conduction slowing (Ref. 7).
An Inspiring Case of Incessant Tachycardia
A 37-year-old man was admitted due to incessant tachycardia. His ECG showed a heart rate of about 150 beats per minute and a complete left bundle branch block (LBBB) pattern. Common medications like lidocaine, verapamil, and propranolol were ineffective. Eventually, his heart rhythm was unexpectedly controlled by right ventricular apical pacing.
A subsequent EP study provided a clear answer. Intracardiac electrograms indicated an AV reentrant tachycardia (AVRT). Its reentrant circuit included:
- The left atrium.
- AV node- His bundle-right bundle branch.
- Right ventricle.
- A left-sided accessory AV bypass tract.
Due to the presence of LBBB, the right ventricle became part of the reentrant circuit, explaining why right ventricular apical pacing could quickly control this persistent tachycardia. Upon careful analysis of the intracardiac electrograms, it was revealed to be a case of concealed WPW syndrome (Ref. 8).
In typical WPW syndrome, there are two conduction pathways between the atria and ventricles: the AV node-His Purkinje system and the accessory AV bypass tract. Serving as the upper and lower common pathways for the atria and ventricles, respectively, they create a natural reentrant circuit that can cause circular movement, leading to AVRT. During normal sinus rhythm, electrical impulses travel from the atria to the ventricles through both pathways, causing a recognizable pattern on the ECG known as ventricular fusion or ventricular preexcitation.
Due to the differences in cellular and tissue structure between these two electrical pathways, they have different electrophysiological properties, such as conduction velocity and refractory period. Thus, after an atrial premature beat (APC), its conduction may be blocked in one pathway and only proceed anterograde through the other pathway, then retrograde through the lower common pathway, re-entering the previously blocked pathway to reach the upper common pathway. The phenomenon of repeating anterograde and retrograde circular conduction can lead to AVRT.
Based on the pathways used for anterograde or retrograde conduction, AVRT can be classified as orthodromic or antidromic. Orthodromic AVRT uses the normal AV node-His Purkinje system for anterograde conduction and the bypass tract for retrograde conduction, producing a narrow QRS complex ECG pattern. Antidromic AVRT uses the opposite pathways, resulting in a wide QRS complex ECG pattern. This is because when the bypass tract is fully involved in anterograde conduction, it causes a complete ventricular preexcitation pattern.
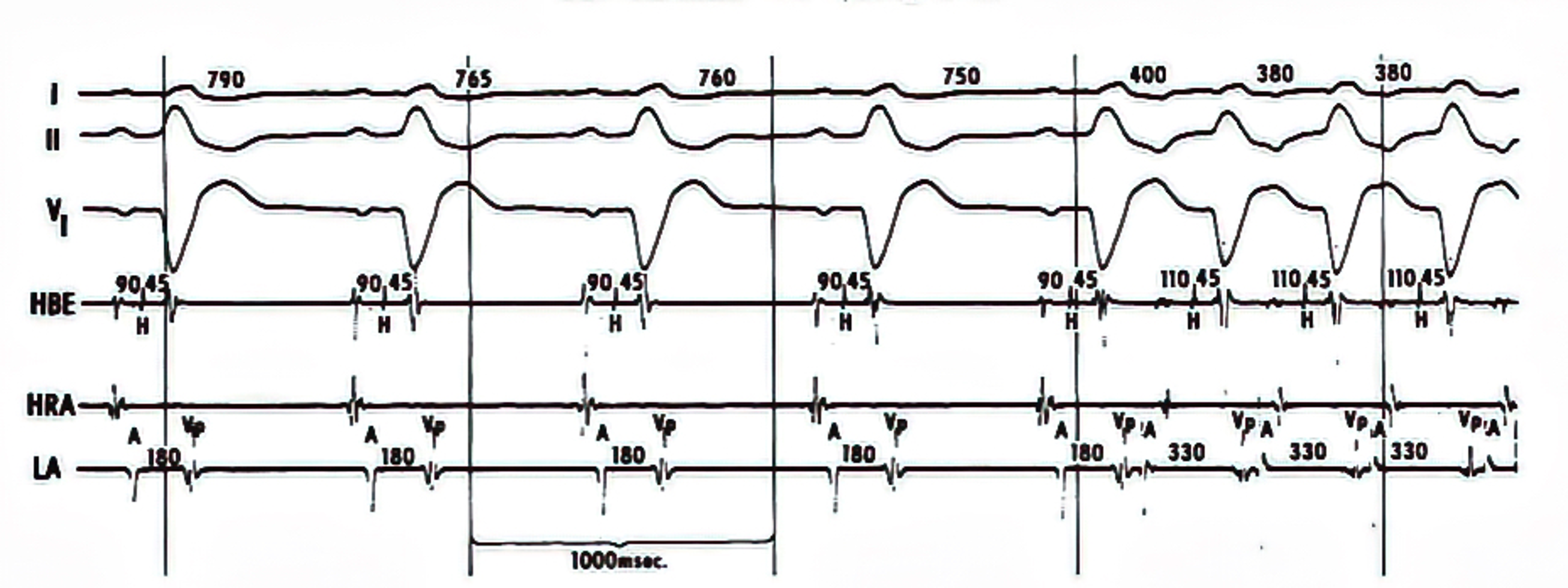
AV reciprocating tachycardia initiated during sinus rhythm without triggering premature atrial or ventricular extrasystole. The atrial (A) - posterobasal LV (Vp) interval of the sinus beat (the 5th QRS complex) recorded from the left atrium (LA, coronary sinus) is 180 msec, the same as those of the preceding sinus beats; however, the gradual shortening of the sinus cycle length (from 790 to 750 msec) is a requisite condition for spontaneous onset of AV reciprocating tachycardia. HBE= His bundle electrogram; HRA= high right atrium (Reference 8, Circulation with permission).
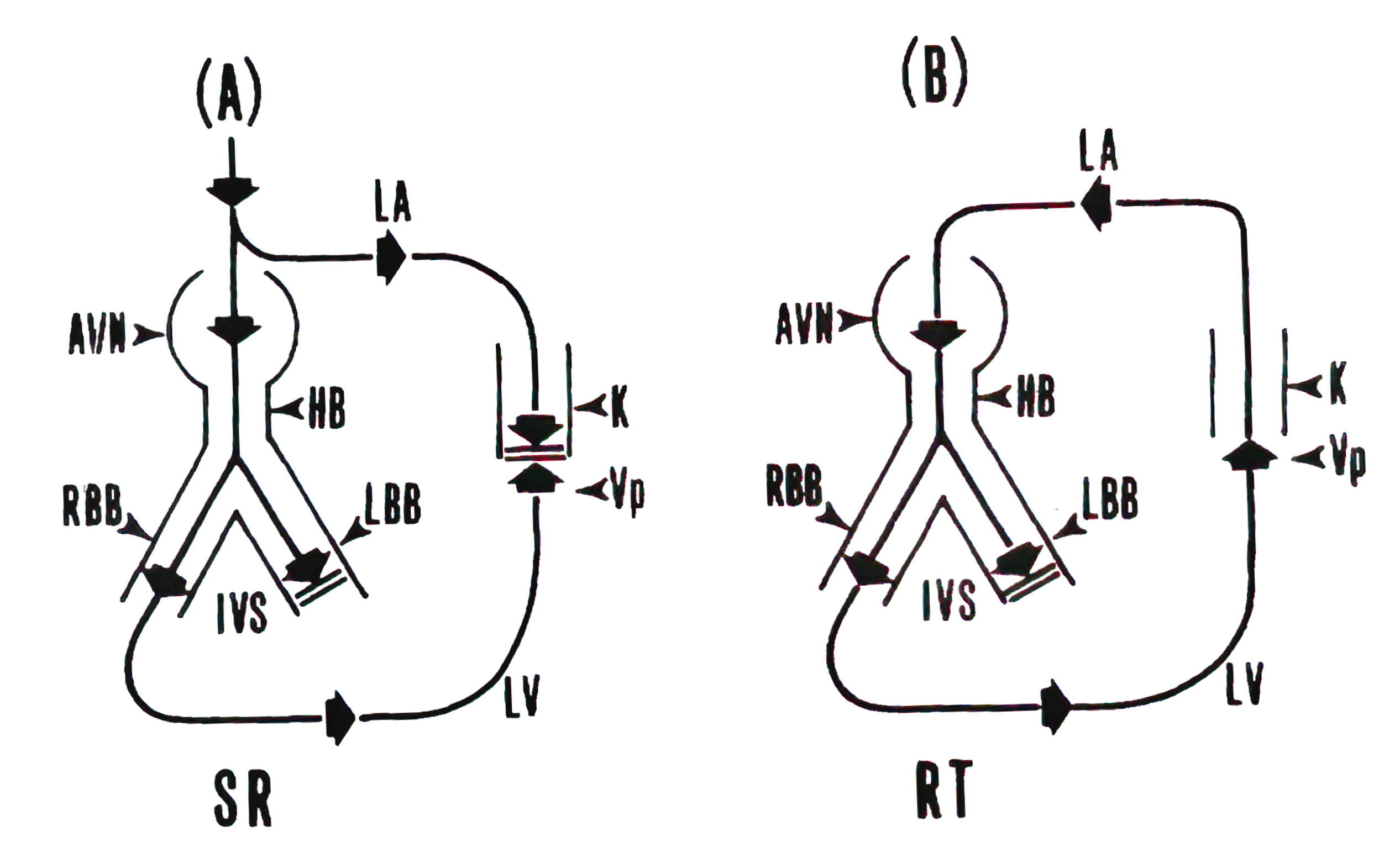
Schematic drawing of the conduction pathways during (A) sinus rhythm (SR) and (B) AV reciprocating tachycardia (AVRT). Because of the antegrade conduction block in the left-sided Kent bundle (K), the impulse proceeds across the atrioventricular node (A VN) and His bundle (HB). In the presence of a rate-dependent conduction block occurring in the left bundle branch (LBB), the impulse propagates through the right bundle branch (RBB) and the right ventricle. It then traverses the interventricular septum (IVS) to activate the left ventricle (LV). An appropriate interplay of impulse arrival time at the posterobasal LV (Vp) and the refractory period of the Kent bundle and the left atrium (LA) would allow the impulse to enter those structures retrogradely, establishing the AVRT circuit (Reference 8, Circulation with permission).
Concealed WPW syndrome
Concealed WPW syndrome has a unique characteristic in that its bypass tract cannot conduct impulses in a forward direction from top to bottom; i.e., it can only conduct in a retrograde or backward manner, known as unidirectional block or capable only of retrograde conduction. In this situation, since the normal electrical impulse is blocked in the bypass tract when it travels downward, no “ventricular fusion” pattern is visible on the ECG during normal rhythm, and atrial premature contractions (APC) are not needed to trigger atrioventricular reentrant tachycardia (AVRT).
Upon analyzing the initiation and termination of AVRT, I found that it always began with a gradual acceleration of normal heart rhythm (decreasing sinus cycle length). Additionally, the rate-dependent left bundle branch block (LBBB) facilitated and supported reentrant circus movement. I realized that the occurrence of AVRT could be explained by basic scientific knowledge: a shortening sinus cycle length reduces the refractory periods of the atria, ventricles, and bypass tract. It may also decrease conduction velocity within the atrioventricular (AN) node, both of which promote and maintain reentrant movement.
In this patient, the bypass tract was located on the left side of the heart. Hence, the occurrence of rate-dependent LBBB further increased the length of the reentrant circuit, resulting in relatively slow conduction and allowing the reentry mechanism to be sustained. Interestingly, the occurrence of rate-dependent LBBB also incorporated the right ventricle into the reentrant circuit, making right ventricular apex pacing (RVA pacing) capable of quickly interrupting the reentrant loop and thereby controlling the heart rhythm.
I first shared my theory with Tino and then reported it at the weekly cardiology department meeting. Later, I presented a talk at the American Heart Association annual meeting with an engaging title- “Mechanism of reciprocating tachycardia initiated during sinus rhythm in concealed Wolff-Parkinson-White syndrome,” which received positive feedback. In 1976, the research paper detailing this study was published in the journal Circulation, where I elaborated on the novel electrophysiological mechanisms involved (Ref. 8).
Although the initiation pattern of this atrioventricular reentrant tachycardia was similar to the permanent junctional reentrant tachycardia (PJRT) described by French clinical electrophysiologist Philippe Coumel in 1975, I was the first to explain the mechanism clearly. From this case, I learned that to study clinical electrophysiology, one must first have a solid understanding of the basic sciences (cellular and tissue) of electrophysiology. Encouraged by this achievement, I continued researching concealed WPW syndrome. I collected a group of adult and pediatric patients with this condition and conducted a comprehensive analysis (Refs. 9,10). The findings from this study involving 12 patients were accepted by the American Journal of Cardiology and published as the leading article in December 1977 (Ref. 10).
Notably, in general, the refractory period of the cells or tissues has two phases: the absolute refractory period and the relative refractory period. Only during the absolute refractory period is it entirely unexcitable (no conduction response). Furthermore, the characteristics of retrograde conduction should not be equated to anterograde conduction; conduction velocity is not solely determined by the refractory period.
Concealed WPW syndrome, a condition where the AV bypass tract lacks anterograde conduction, thus masking the ECG signs of ventricular preexcitation, is a significant area of study. The term “concealed conduction” refers to the effect of a nonconducted (unsuccessfully conducted) impulse on subsequent impulse conduction, a phenomenon most pronounced in the AV node. This node, with its reliance on calcium channels and its slow conduction characteristics, is a crucial area of study. Our research has shown that this phenomenon is not limited to the AV node but can also be observed in other tissues, such as human atria and bypass tracts, highlighting the relevance of our work to various areas of electrophysiology (Refs. 11,12).
Overcoming Limitations and Breaking through
Due to the lack of tape-recording equipment in the catheterization lab, we often missed key events displayed on the screen during episodes of cardiac arrhythmias. To capture everything shown on the screen, I continuously printed out hard copies, resulting in a large volume of data and increased costs. However, this method provided me with the opportunity to conduct detailed analyses. Taking WPW syndrome as an example, this approach allowed me to identify the characteristics of orthodromic and antidromic conduction as well as the initiation and termination of atrioventricular reentrant tachycardia (AVRT), leading to the completion of a paper with systematic interpretations of the findings. (Ref. 13).
This extended series of papers also yielded a serendipitous discovery. I recorded the spontaneous alternation between atrial fibrillation (AF) and atrioventricular (AV) reciprocating tachycardia in patients with WPW syndrome. An atrial premature beat usually triggers the spontaneous conversion of AV reciprocation to AF. In contrast, spontaneous conversion of AF to AV reciprocation requires the development of anterograde unidirectional block in the accessory bypass tract. These findings were published in Circulation in 1977 (Ref. 14). Although it would take time to elucidate the potential mechanisms behind these transitions, this article remains one of my favorites to discuss with my students.
Dual Atrioventricular [AV] Nodal Pathway Physiology
At the time, in addition to atrioventricular reentrant tachycardia (AVRT) in Wolff-Parkinson-White (WPW) syndrome, atrioventricular nodal reentrant tachycardia (AVNRT) in the general population was also a frequent topic of research and discussion. AVNRT is a common arrhythmia that was first described in 1915. The team led by Professor Kenneth Rosen at the University of Illinois made significant contributions to the study of this arrhythmia. In the EP study lab, they used two sets of atrioventricular nodal conduction intervals (A-H interval) to identify the existence of dual AV nodal pathway conduction. They believed that the proximal common pathway and distal common pathway together form the reentrant circuit. They also hypothesized that functional dissociation was the underlying mechanism of dual AV nodal pathway conduction. I learned a lot from their lectures and publications. Still, the human AV node is tiny (compact node: 1 x 3 x 5 mm3), making it difficult for me to understand the abstract concept of functional dissociation.
One morning, I excitedly shared with Tino my belief that I had identified the clinical equivalent of the reverse form of AVNRT observed in Gordon Moe’s 1956 animal experiments (dogs and rabbits). To my surprise, Tino appeared unmoved and presented me with a manuscript that he was reviewing for Circulation. It was a paper by Delon Wu of Kenneth Rosen’s group describing three cases of an “unusual variety of AV nodal reentrant tachycardia.” Although I was disappointed not to be the first to make this discovery, I was undeterred.
The “Slow-Fast” and “Fast-Slow” Forms of AV Nodal Reentrant Tachycardia
Subsequently, I meticulously analyzed our own AVNRT cases to reveal how programmed atrial and ventricular stimulation induce the typical and reversed forms of AVNRT at different pacing cycle lengths (400ms and 600ms). I submitted the findings to the American Journal of Cardiology (Ref. 15). The recognition from one of the reviewers, likely Professor Kenneth Rosen, was significant. His positive feedback, acknowledging that our findings corroborated some of his group’s observations, was a validation of our work. Rosen’s suggestion to use the now widely used terminology of ‘slow-fast’ and ‘fast-slow’ rather than ‘usual’ and ‘unusual’ for the two forms of AVNRT further underscored the significance of our research. Ken Rosen, a well-respected academic leader in his late 30s, known for his friendly and enthusiastic personality, invited me to join his team at the University of Illinois in Chicago when a position opened. It was a tempting offer, but ultimately, I decided to stay with Tino and continue my research work at the University of Miami.
Later, I also observed an intricate electrophysiologic phenomenon in which dual AV nodal pathway conduction occurred in patients with accessory AV bypass tracts (Ref. 16). My growing reputation led to significant national and international recognition. I was invited to chair and speak at conferences on supraventricular tachycardias, focusing on diagnosis and management. Circulation and the American Journal of Cardiology sought my expertise as a reviewer for clinical electrophysiology articles. The American Heart Journal appointed me to their Editorial Board. These travels allowed me to meet prominent electrophysiologists worldwide, including Philippe Coumel and Paul Peuch (France), RM Schuilenberg, Heins J. J. Wellens, Michael Janse, and Dick Durrer (Netherlands), David Ross (Australia), Joseph Brugada (Spain), and Alfred Waldo, Onkar Narula, Pablo Dennis, Delon Wu, John Gallagher, Douglas Zipes, Andrew L. Wit and Mark E. Josephson (US). The frequent travel also resulted in my achieving ” Premier Gold” status with United Airlines, offering some perks to ease the journey.
The Department of Medicine acknowledged my contributions to establishing UM’s reputation in clinical cardiac electrophysiology, both nationally and internationally. This recognition led to my promotion to Associate Professor in 1978, a significant milestone in my professional growth and a testament to the impact of my work.
A Pacemaker Innovation.
Tino and Barold V. Berkovits, a brilliant electrical engineer at Cordis Corporation in Miami, collaborated on a project to develop a specialized implantable pacemaker for bradycardia pacing. Their vision was a pacemaker capable of sequentially synchronized stimulation of the atrium and ventricle. It later became the dual-chamber atrial and ventricular physiologic pacemaker.
Along the way, Tino and I discussed the possibility of using simultaneous atrial and ventricular stimulation to disrupt the initiation of atrioventricular nodal reentrant tachycardia (AVNRT) triggered by premature atrial or ventricular beats. To paraphrase, introducing an “impulse collision” within the AV node with each simultaneous atrial and ventricular pacing could prevent the initiation of reentry. I then took the initiative to start a first clinical trial. The study successfully eliminated the inducible tachycardia zone in four patients using programmed pacing (Ref. 17). However, as we enrolled more patients, the influence of the dynamic autonomic nervous system became apparent. Its impact on AV node function could eventually alter the suppressive effect of simultaneous atrial and ventricular stimulation, allowing reentry and arrhythmia to occur (unpublished data). Despite this initial setback, I learned that the dynamic nature of the autonomic nervous system should always be considered in the management of patients with cardiac arrhythmias.
Amiodarone, Verapamil, and N-acetyl procainamide as Antiarrhythmic Agents
In exploring new frontiers in arrhythmia management, Europe and South America had a greater latitude to experiment with new drugs. In 1974, Dr. Mauricio Rosenbaum (1921-2003), a prominent Argentine cardiologist, visited Tino and introduced us to amiodarone, an experimental medicine at the time. I began using amiodarone in the CCU for patients with treatment-resistant arrhythmias, primarily ventricular tachyarrhythmias. It was my first experience with a drug with such a long half-life of up to three months. Later experience also revealed the potential for multiple side effects associated with this powerful antiarrhythmic drug- hepatotoxicity, corneal deposit, pseudocyesis (skin photosensitivity), thyroid dysfunction, neurotoxicity, pneumonitis, pulmonary fibrosis, etc.
My journey of exploration led me to study verapamil in its intravenous form, marking the beginning of pharmacological investigations during the EP study. Verapamil, a member of the phenylalkylamine class of calcium channel blockers, showed promising potential. Its mechanism of action, suppressing AV node conduction, proved to be a beacon of hope in interrupting supraventricular tachycardia (SVT) that relies on the AV node as part of the reentrant circuit. Our EP study revealed that verapamil effectively suppressed sinus node and atrioventricular node reentrant tachycardias (AVNRT) and slowed the ventricular response during atrial flutter and fibrillation (Refs. 18,19).
Our journey of discovery also led us to study N-acetyl procainamide, the active metabolite of procainamide. This compound possesses an antiarrhythmic mechanism by selectively lengthening the action potential duration (QT interval) (Vaughan-Williams Class III antiarrhythmic action). It initially looked promising because it does not cause lupus syndrome, unlike the parent drug procainamide. However, our EP study revealed a sobering truth- its antiarrhythmic efficacy might not be as potent as procainamide (Ref. 20). This finding, while not as exciting as we had hoped, was an integral part of our journey in arrhythmia management.
The Pressure to Publish is a Constant Reality in Academic Medicine.
The phrase “publish or perish” aptly captures this challenge. Researchers need a steady stream of ideas to keep their research protocols moving forward. Fortunately, professional meetings provide a valuable platform. Annual meetings of the American Heart Association, the American College of Cardiology, and the North American Society of Pacing and Electrophysiology (Heart Rhythm Society) allow researchers to share their preliminary findings through abstracts. These meetings foster collaboration and a vibrant exchange of knowledge within cardiology subspecialties. While I may not have artistic talents such as singing or painting, God has blessed me with a curious mind and the perseverance to see projects through. From 1975, when I joined the faculty, until I transitioned to a purely educational and administrative role in 2001, this flow of ideas fueled my research endeavors. Witnessing the remarkable advances in cardiology during this time has been truly rewarding. To name a few, I have seen the development of physiologic pacing, the advent of balloon angioplasty and coronary stenting, the refinement of dual chamber pacemakers and implantable cardioverter-defibrillators, and the introduction of new medications for hypertension, hyperlipidemia, arrhythmias, and heart failure.
The demands of a “triple-threats” faculty role were considerable. In the 1960s and 1970s, medical schools often sought faculty who excelled in all three areas: conducting original research, publishing prolifically, and being both inspiring educators and compassionate practicing physicians. Undeniably, this placed immense pressure on individuals, myself included. To relax, I enjoyed spending time with my family, attending gatherings of Taiwanese compatriots, and playing tennis. Tsu-Ming Lin (林祖銘) and his wife were close friends of Kuei and me. Tsu-Ming became my regular tennis partner, and tennis and movies were my stress relievers.

Attending the 10th Annual Meeting of the Interamerican Congress of Cardiology in Caracas, Venezuela From the left, Dr. and Mrs. Stephen Mallon and Dr. and Mrs. Cesar Conte. On the far right is Dr. Juan Aranda.
A Chance Encounter during a Routine Procedure Sparked the Advancement of Academics.
One day, I placed an additional electrode catheter into the coronary sinus in a patient who had experienced palpitations but no documented arrhythmias. The study revealed no accessory AV bypass tracts but did show dual AV nodal pathway conduction in both the forward (anterograde) and backward (retrograde) directions. The patient also had inducible ventricular echoes. This latter phenomenon shows that ventricular premature stimulation (VPS) can induce retrograde conduction, followed by an atrial response, which is conducted anterograde, leading to another ventricular response, indicating the presence of dual AV nodal pathway conduction.
While I was reviewing the recordings at home, a fascinating detail emerged. The multiple atrial electrograms displaced a distinct shift in the backward atrial activation sequence during the transition between fast and slow retrograde conduction pathways. The consistency of this shift across multiple recordings compelled me to take a closer look. Excitement grew as I realized that I may have stumbled upon the anatomical basis for the physiology of dual AV nodal conduction in humans. Based on the retrograde atrial activation sequence, I hypothesized that the fast pathway lies anteriorly near the septum (where His bundle activity is recorded). In contrast, the slow pathway was posteriorly located near the front of the coronary sinus orifice. This discovery mirrored observations in rabbit hearts by Mendez and Moe (Ref. 21) and Janse et al. (Ref. 22). More importantly, it indicated that AV nodal reentry in humans wasn’t just a “functional dissociation” but had an anatomic structural basis.
To confirm these findings, I sought additional patients. In collaboration with cardiology fellows, I identified six additional patients who underwent electrophysiological studies and demonstrated dual AV nodal pathway conduction in both directions. The consistent presence of the retrograde atrial activation shift in these patients increased my confidence in the anatomic basis of dual AV nodal pathway physiology and AVNRT in humans.
Anteroposterior view of intracardiac catheter electrode positions under fluoroscope. The two coronary sinus leads (CS1 and CS2) are 10 mm apart and are located underneath the His bundle electrographic lead (HBE). The CS1 lead is believed to be at the CS ostium. HRA = high right atrium; LRA = lateral right atrium; RVA = right ventricular apex; SRA = low septal right atrium (Reference 23, Circulation with permission).
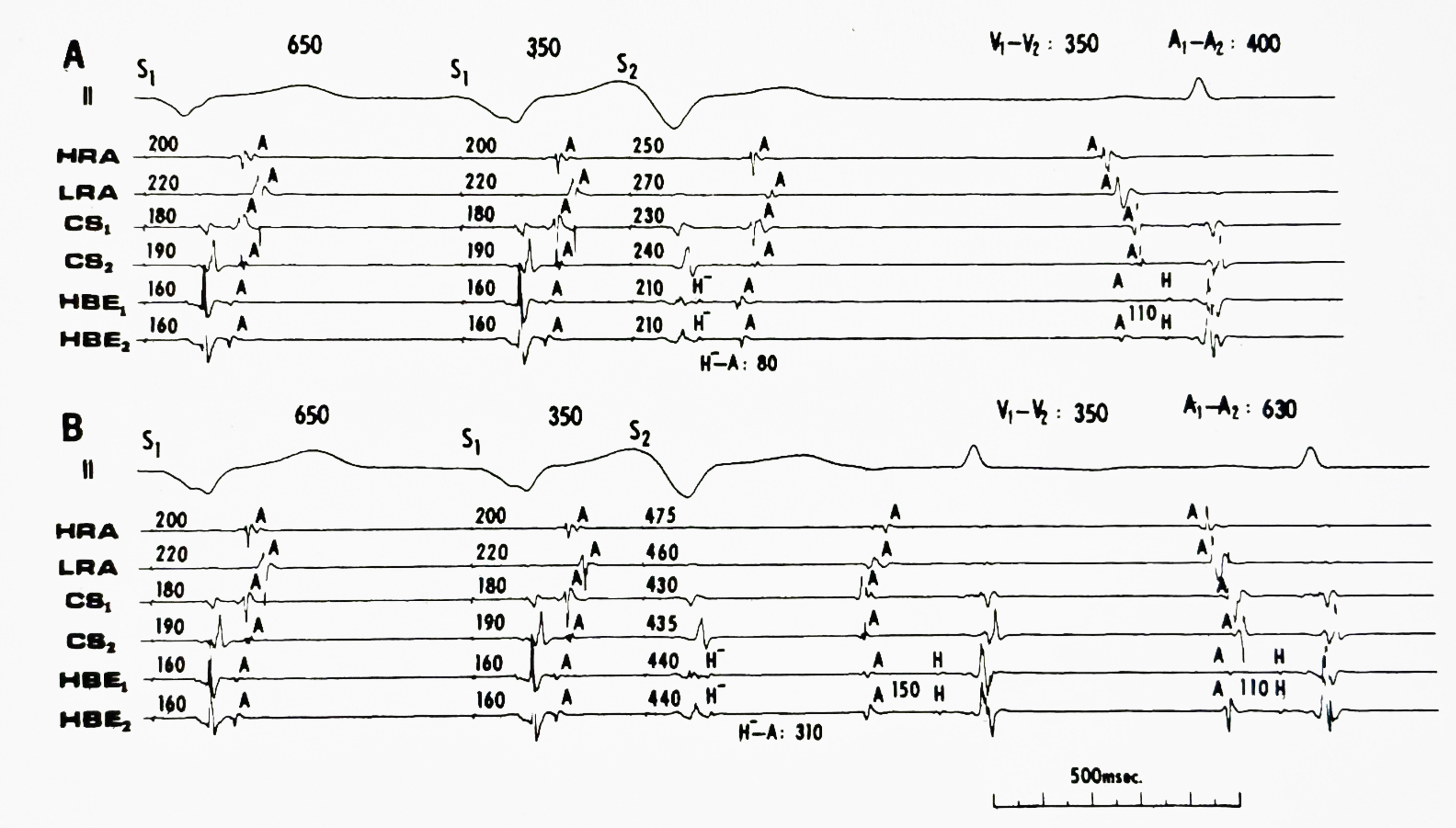
Sharing the Discovery: Initial Resistance and Eventual Acceptance. In 1979, I presented the anatomic basis for AV nodal reentry for the first time as an abstract at the American Heart Association's annual meeting. I was privileged to see such luminaries as Kenneth Rosen, Alfred Waldo, and Dirk Durrer listen to me. After that, I immediately submitted the manuscript to Circulation magazine for publication. The initial reviews were mixed. One reviewer provided valuable support, while the other raised concerns about potential artifacts due to catheter movement during cardiac contraction and relaxation. The critical tone suggested reviewer might be Dr. John Gallagher, a renowned WPW expert from Duke University. His criticism mirrored his previous dismissal of my paper on spontaneous conversion between AF and atrioventricular reciprocating tachycardia in WPW syndrome (Ref. 14), a work I highly valued. Dr. Gallagher had called those results "trivial." Based on my experience, my courageous appeal to the editor, while responding in detail to the comments of two reviewers, resulted in the editor inviting a third reviewer for arbitration. The journal Circulation finally accepted the paper (Ref. 23).
A Chance Encounter during a Routine Procedure Sparked the Advancement of Academics.
One day, I placed an additional electrode catheter into the coronary sinus in a patient who had experienced palpitations but no documented arrhythmias. The study revealed no accessory AV bypass tracts but did show dual AV nodal pathway conduction in both the forward (anterograde) and backward (retrograde) directions. The patient also had inducible ventricular echoes. This latter phenomenon shows that ventricular premature stimulation (VPS) can induce retrograde conduction, followed by an atrial response, which is conducted anterograde, leading to another ventricular response, indicating the presence of dual AV nodal pathway conduction.
While I was reviewing the recordings at home, a fascinating detail emerged. The multiple atrial electrograms displaced a distinct shift in the backward atrial activation sequence during the transition between fast and slow retrograde conduction pathways. The consistency of this shift across multiple recordings compelled me to take a closer look. Excitement grew as I realized that I may have stumbled upon the anatomical basis for the physiology of dual AV nodal conduction in humans. Based on the retrograde atrial activation sequence, I hypothesized that the fast pathway lies anteriorly near the septum (where His bundle activity is recorded). In contrast, the slow pathway was posteriorly located near the front of the coronary sinus orifice. This discovery mirrored observations in rabbit hearts by Mendez and Moe (Ref. 21) and Janse et al. (Ref. 22). More importantly, it indicated that AV nodal reentry in humans wasn’t just a “functional dissociation” but had an anatomic structural basis.
To confirm these findings, I sought additional patients. In collaboration with cardiology fellows, I identified six additional patients who underwent electrophysiological studies and demonstrated dual AV nodal pathway conduction in both directions. The consistent presence of the retrograde atrial activation shift in these patients increased my confidence in the anatomic basis of dual AV nodal pathway physiology and AVNRT in humans.
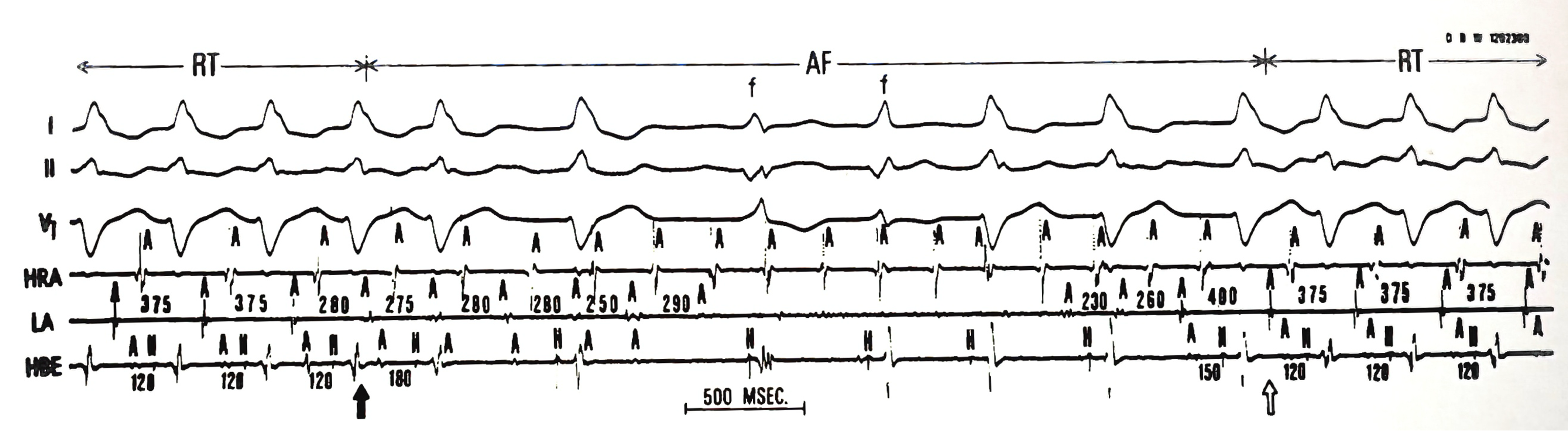
Spontaneous conversion between AV reciprocating tachycardia (AVRT) and atrial flutter-fibrillation (AF) in a patient with a left-sided AV bypass tract. The left atrial depolarization (A-A interval of 280 msec) following the fourth QRS complex (solid arrow) is premature with respect to the expected reciprocating rhythm (A-A interval of 375 msec) during AVRT. The corresponding A-H interval is lengthened from 120 msec to 180 msec following the atrial premature depolarization. A short run of AF with a mean atrial cycle length of 240 msec is then initiated. During AF, the atrial electrograms are fairly regular in the HRA lead but are fractionated in both LA and HBE leads. The seventh and eighth QRS complexes are fusion beats (f), as evidenced by the inscription of delta wave (ventricular pre-excitation) before the His bundle potential (H). The eleventh QRS complex is the last AF conducted beat, which then enters the left atrium (hollow arrow), initiating AVRT. Note prior to the conversion of AF to AVRT, the last three AF-conducted beats (ninth, tenth, and eleventh QRS complexes) are anterogradely blocked in the anomalous pathway as evidenced by the disappearance of the delta wave and normalization of the H- V interval (50 msec). A complete left bundle branch block pattern is present during AVRT (Reference 14, Circulation with permission).
Over the years, the concept of anatomic AV nodal reentry has gained considerable traction in clinical cardiac electrophysiology. However, it wasn’t until 1992 that Warren Jackman at the University of Oklahoma successfully translated this research into a therapeutic approach. Using radiofrequency ablation to target slow pathway conduction near the coronary sinus ostium, Jackman successfully cured AV nodal reentrant tachycardia in 78 patients (Ref. 24). Because the slow pathway conduction zone is located approximately 1.3 cm below the His bundle, it has become the preferred site for catheter ablation to cure AVNRT (Ref. 25).
My research productivity flourished at the University of Miami (1974-1981). The academic reputation led to numerous invitations from the American Heart Association, American College of Cardiology, and Heart Rhythm Society to participate in lectures and symposia on the physiology of the human dual AV nodal pathway conduction and AVNRT ablation techniques. One particularly memorable moment was when Dr. John Gallagher of Duke University publicly acknowledged that he was the reviewer who initially rejected my paper on anatomic AV nodal reentry for publication. He earned my respect by formally apologizing before introducing me as a speaker at a symposium. John Gallagher’s public apology to me not only eliminated my previous dissatisfaction with him (for sharply criticizing two essential articles of mine) but also gained my admiration. I have always thought that this kind of demeanor is something that many Taiwanese people must learn.
New Findings Spark Debate on AV Node Definition
The successful radiofrequency ablation of AVNRT validated the anatomic concept of AV nodal reentry, but it also ignited a debate on how we define the human AV node. In human hearts, Tawara divided the AV node into a compact node and a lower nodal bundle with extensions toward the coronary sinus without specifying their functionality (Ref. 26). In isolated rabbit hearts, Janse et al. demonstrated dual AV nodal inputs and induction of atrial echo phenomenon with atrial extra stimulation (Ref. 22). Unfortunately, they could not wholly map out the AV nodal reentrant circuit and suggested that many possible different pathways might exist within the AV node. Fundamental questions have since emerged: is the slow pathway truly an extranodal structure, or is it simply part of the posterior extension of the AV node? Additionally, where should ablation be targeted – the fast or slow pathway? Some researchers even posit that dual pathway conduction might not involve distinct pathways at all but instead arise from inhomogeneous conduction within the Koch triangle, a well-defined anatomic region at the base of the right atrium. Despite ongoing vigorous discussions, selective ablation of the slow pathway conduction remains the preferred approach due to its lower risk of unintended AV block and complications that could promote arrhythmias (Ref. 25).
Nearly a Decade in Miami and the Decision to Move to San Francisco
My productive research career at UM led to several opportunities at other institutions. With Tino’s encouragement and guidance, I carefully considered these offers and visited five medical centers between 1979 and 1980. The University of Colorado offered the most enticing package, with immediate promotion to full professor of medicine and chief of cardiology at Denver General Hospital. Although tempting, I needed more experience for such a senior position. Instead, I decided to join Melvin M. Scheinman’s group at the University of California at San Francisco (UCSF) as an associate professor.
Several factors drew me to the University of California, San Francisco (UCSF). UCSF consistently ranked among the top five medical schools in the United States, on par with Harvard University, Johns Hopkins University, and Stanford University School of Medicine. San Francisco had a vibrant and multicultural atmosphere with a large Asian community. Additionally, its proximity to Taiwan, with frequent direct flights, made it convenient for me to return to my home country. Lastly, the fact that my high school classmate Shin Wu (吳新一) lived in Cupertino, near the San Francisco Bay Area, was particularly appealing.
News of my imminent departure reached Dr. Daniel H. Mintz (1930-2020), Chair of Internal Medicine at the University of Miami. He visited my wife, Kuei, and me and urged us to reconsider. Learning I was considered one of Miami’s three ‘academic stars’ was a humbling honor. Dr. Mintz’s full support for my retention was deeply appreciated, and I will always be grateful for his guidance. However, a yearning for independence had taken root. Under Tino’s tutelage, I transitioned from interpreting electrocardiograms (static electrophysiology) to delving into the mechanisms of arrhythmias (dynamic electrophysiology). I felt prepared to forge my own academic path. Additionally, I also wanted the freedom to publish my work without having to list my superiors as co-authors.
A Valuable Warning: at Crossroads- Prestige vs. Promotion
Interestingly, upon learning of my decision to join UCSF, Dr. Shahbudin Rahimtoola (1931-2018), Chief of Cardiology at the University of Southern California (USC), contacted me. He expressed concern that the move would be a lateral transfer, unlikely to lead to a full professorship. In contrast, USC had also offered me a promotion to full professorship. He also cautioned against working under someone who had yet to be established as a leading electrophysiologist, along with a possible reduction in salary. These were valid points, but the allure of UCSF, a medical powerhouse with an illustrious global reputation, proved irresistible. The chance to collaborate with luminaries like Lloyd Hollingsworth ‘Holly’ Smith Jr. (1924-2018), chair of UCSF’s Department of Medicine, and Dr. William Parmley, Chief of Cardiology, was an honor I couldn’t pass up, and I was filled with excitement about the prospect.
References:
(2) Sung RJ, Ghahramani AI, Mallon SM, Richter SE, Sommer LS, Gottlieb S, Myerburg RJ. Hemodynamic features of prolapsing and non-prolapsing left atrial myxoma. Circulation 1975;51:342-349.
(3) Sung RJ, Mallon SM, Richter SE, Ghahramani AR, Sommer LS, Kaiser GA, Myerburg RJ. Left main coronary artery obstruction. Follow-up of thirty patients with and without surgery. Circulation, 1975;52 (Suppl I):112-118.
(4) Scherlag BJ, Lau SH, Helfant RH, Berkowitz WD, Stein E, Damato AN: Catheter technique for recording His bundle activity in man. Circulation, 1969;39:13-18.
(5) Sung RJ, Tamer DM, Agha AS, Castellanos A, Myerburg RJ, Telband H. Etiology of the electrocardiographic pattern of “incomplete right bundle branch block” in atrial septal defect: An electrophysiologic study. Journal of Pediatrics, 1975;87:1182-1186.
(6) Sung RJ, Tamer DM, Garcia OL, Castellanos A, Myerburg RJ, Gelband H. Analysis of surgically induced right bundle branch block pattern using intracardiac recording techniques. Circulation, 1976;54:442-446.
(7) Mines GR. On dynamic equilibrium in the heart. Journal of Physiology, 1913;46:349-383.
(8) Sung RJ, Castellanos A, Gelband H, Myerburg RJ. Mechanism of reciprocating tachycardia initiated during sinus rhythm in concealed Wolff-Parkinson-White syndrome: Circulation, 1976;54:338-345.
(9) Sung RJ, Ferrer P, Gardia OL, Castellanos A, Gelband H. Atrioventricular reciprocal rhythm and chronic reciprocating tachycardia in a newborn infant with concealed Wolff-Parkinson-White syndrome. British Heart Journal, 1977;39:810-814.
(10) Sung RJ, Gelband H, Castellanos A, Aranda JM, Myerburg RJ. Clinical and electrophysiological observations in patients with concealed accessory bypass tracts. American Journal of Cardiology, 1977;40:839-847.
(11) Sung RJ, Myerburg RJ, Castellanos A. Electrophysiologic demonstration of concealed conduction in the human atrium. Circulation, 1978;58:940-6.
(12) Svinarich JT, Tai DY, Mickelson J, Keung EC, Sung RJ. Electrophysiologic demonstration of concealed conduction in anomalous atrioventricular bypass tracts. Journal of American College of Cardiology, 1985;5:898-903.
(13) Sung RJ, Castellanos A, Mallon SM, Gelband H, Mendoze I, Myerburg RJ. The mode of initiation of reciprocating tachycardia during programmed ventricular stimulation in the Wolff-Parkinson-White syndrome. With reference to various patterns of ventriculoatrial conduction. American Journal of Cardiology, 1977;40:24-31.
(14) Sung RJ, Castellanos A, Mallon SM, Bloom MG, Myerburg RJ. Mechanisms of spontaneous alternation between reciprocating tachycardia and atrial flutter-fibrillation in the Wolff-Parkinson-White syndrome. Circulation, 1977;56:409-416.
(15) Sung RJ, Styperek JL, Myerburg RJ, Castellanos A. Initiation of two distinct forms of atrioventricular nodal reentrant tachycardia during programmed ventricular stimulation in man. American Journal of Cardiology, 1978;42:404-415.
(16) Sung RJ, Styperek JL. Electrophysiologic identification of dual atrioventricular nodal pathway conduction in patients with accessory bypass tracts. Circulation, 1979;60:1464-1476.
(17) Sung RJ, Styperek JL, Castellanos A. Complete abolition of the reentrant supraventricular tachycardia zone using a new modality of cardiac pacing with simultaneous atrioventricular stimulation. American Journal of Cardiology, 1980;45:72-78.
(18) Sung RJ, Elser B, McAllister RG. Intravenous Verapamil for termination of reentrant supraventricular tachycardia: intracardiac studies correlated with plasma verapamil concentrations. Annals of Internal Medicine, 1980;93:682-689.
(19) Waxman HL, Myerburg RJ, Appel R, Sung RJ. Verapamil for control of ventricular rate in paroxysmal supraventricular tachycardia and atrial fibrillation or flutter: a double-blind randomized cross-over study. Annals of Internal Medicine, 1981;94:1-6.
(20) Sung RJ, Juma Z, Saksena S. Electrophysiologic properties and antiarrhythmic mechanisms of intravenous N-acetyl procainamide in patients with ventricular dysrhythmias. American Heart Journal, 1983;105:811-823.
(21) Mendez C, Moe GK. Demonstration of a dual A-V nodal conduction system in the isolated rabbit heart. Circulation Research, 1966;19:378-93.
(22) Janse MJ, Van Capelle FJL, Anderson RH, Touboul P, Billette J: Electrophysiology and structure of the atrioventricular node of the isolated rabbit heart. In The Conduction System of the Heart, edited by Wellens HJJ, Lie K, Janse M. Leiden, Stenfert Kroese, 1976, p 296.
(23) Sung RJ, Waxman HL, Saksena S, Juma Z. Sequence of retrograde atrial activation in patients with dual atrioventricular nodal pathways. Circulation, 1981;64:1059-1067.
(24) Jackman WM, Beckman KJ, McClelland JH, Wang X, Friday KJ, Roman CA, Moulton KP, Twidale N, Hazlitt HA, Prior MI, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. New England Journal of Medicine, 1992;327:313-318.
(25) Fundamental Approaches to the Management of Cardiac Arrhythmias by Ruey J. and Michael R. Lauer. In Chapter 11, Atrioventricular nodal reentrant tachycardia, pp 488-509 in Kluwer Academic Publishers, Dordrecht/Boston/London, 2000.
(26) Tawara S. Das Reizleitungssystem des Saugetierherzens: Eine Anatomisch-Histologische Studie Uber Das Atrioventrikularbundel Und Die Purkinjeschen Faden. Jena: Verlag von Gustav Fischer; 1906.
University of California at San Francisco (UCSF) (March 1, 1981-February 28, 1991)
Life in San Francisco: Beauty and Challenges
The University of California, San Francisco (UCSF) offered a new chapter, both personally and professionally. Nestled near the iconic Golden Gate Bridge and Golden Gate Park, UCSF’s location provided a vibrant backdrop for our lives. Kuei and I found a home in a quiet suburban neighborhood.
UCSF’s medical school boasted a remarkable faculty. In cardiology, names like Kanu Chatterjee and William W. Parmley (heart failure), Nelson Schiller (echocardiography), Elias Botvinick (nuclear imaging cardiology), and Melvin Cheitlin and Elliot Rappaport (clinical cardiology) were among the academic stars. Initially, working alongside such revered colleagues made me feel like a ‘rabbit in the headlights’. However, after I settled in, I was honored to be invited to join the editorial board of the Journal of the American College of Cardiology, a position I had long aspired to.
I initially started my career at Moffitt-Long Hospital, UCSF’s primary affiliated medical center. This placement presented me with unexpected challenges. As I was new to the area, I received few referrals from patients and, therefore, often ended up doing treatment work only for patients of Professor Melvin Scheinman. Although Melvin Scheinman was gracious, his approach to patient care frequently differed from mine. In retrospect, Professor Shahbudin Rahimtoola at USC was right in his warning about working under someone with limited autonomy.
Seeking Opportunities and Building Alliances
Fortunately, UCSF was comprised of three affiliated hospitals: Moffitt-Long, San Francisco General, and the Veterans Administration Hospital. Seeking a better fit, I requested a transfer to San Francisco General Hospital (SFGH) while keeping a private clinic at Moffit-Long. While some colleagues expressed concerns about SFGH’s focus on indigent care and AIDS (acquired immunodeficiency syndrome) research, I remained optimistic. Limited private patient referrals were a reality due to my recent arrival in the region. However, God endowed me with resilience and perseverance, and I held firm to the belief that “where there’s a will, there’s a way.”
While I enjoyed working with Melvin Cheitlin and Elliot Rappaport in the Cardiology round, proactive outreach led me to collaborate with young physicians at nearby medical centers. Edmond Keung, a UCSF assistant professor at the Veterans Administration Hospital (VAH), and Edward Huckye (later, Tom Synarich and Jim Cockrell) from the Letterman Army Hospital Medical Center (LAMC) became vital partners. The three hospitals- SFGH, VAH, and LAMC, located geographically relatively close to each other in the city of San Francisco, formed the foundation for a solid clinical electrophysiology team. Interestingly, LAMC, which catered to military personnel in the western US, was the counterpart of Walter Reed Army Medical Center in the east US (Washington, DC). Both boasted a vast patient source.

1982 at Cardiac Catheterization Laboratory, Letterman Army Hospital, Presidio, San Francisco.

The farewell dinner for Dr. Edward Huycke's promotion and moving to Walter Reed Army Medical Center, Washington, DC, on June 25, 1989.

The farewell dinner for Dr. Edward Huycke's promotion and moving to Walter Reed Army Medical Center, Washington, DC, on June 25, 1989.
A significant shift in research direction to focus on ventricular tachycardia.
Before leaving the University of Miami, I established an independent electrophysiology (EP) laboratory at Jackson Memorial Hospital (JMH) in February 1981. There, I completed my first electrophysiological study case on ventricular tachycardia (VT). Patients with VT typically have underlying structural heart disease, either ischemic or non-ischemic cardiomyopathy. During VT, abnormal depolarization and rapid heart rate often lead to myocardial ischemia, which can quickly deteriorate VT into ventricular fibrillation (VF), leading to death. In this regard, Mark E. Josephson from the University of Pennsylvania pioneered electrophysiological mapping at surgery for VT.
At UCSF, I resumed academic pursuits and published articles on previous research interests (Refs. 27-31). However, I soon renewed my interest in VT. My primary focus shifted to VT and VF in relation to sudden cardiac death (Ref. 32), digging into their underlying mechanisms across various clinical contexts.
Isoproterenol Infusion Studies
Having observed how “exercise” could trigger arrhythmias, I administered isoproterenol to patients to simulate this phenomenon, defining the resulting arrhythmias as catecholamine-sensitive. Isoproterenol, structurally similar to epinephrine, was administered intravenously as a bolus (1-2 mcg) followed by a continuous infusion (0.15-0.3 mcg/minute) or titrated to maintain a 25% increase in sinus rate from baseline. Using this approach, I successfully induced “exercise”-related atrial tachycardia, AV nodal reentrant tachycardia, atrial flutter, atrial fibrillation, and VT (Refs. 33-35). Our electrophysiology team presented our findings at national and international conferences many times.
Subsequently, after analyzing the data collected from EP studies of VT patients, I examined the initiation and termination patterns of VT, as well as its responses to verapamil (a calcium channel blocker) and propranolol (a beta-blocker). This analysis led to the classification of VT into three distinct categories based on the mechanisms described in cellular electrophysiology. These categories were reentry, enhanced automaticity, and triggered activity. Each category represented a different underlying mechanism of VT, and understanding these mechanisms was crucial for developing effective treatment strategies. These findings were published in the prestigious Journal of Clinical Investigation (JCI) in 1983 and 1988 (Refs. 37,38).
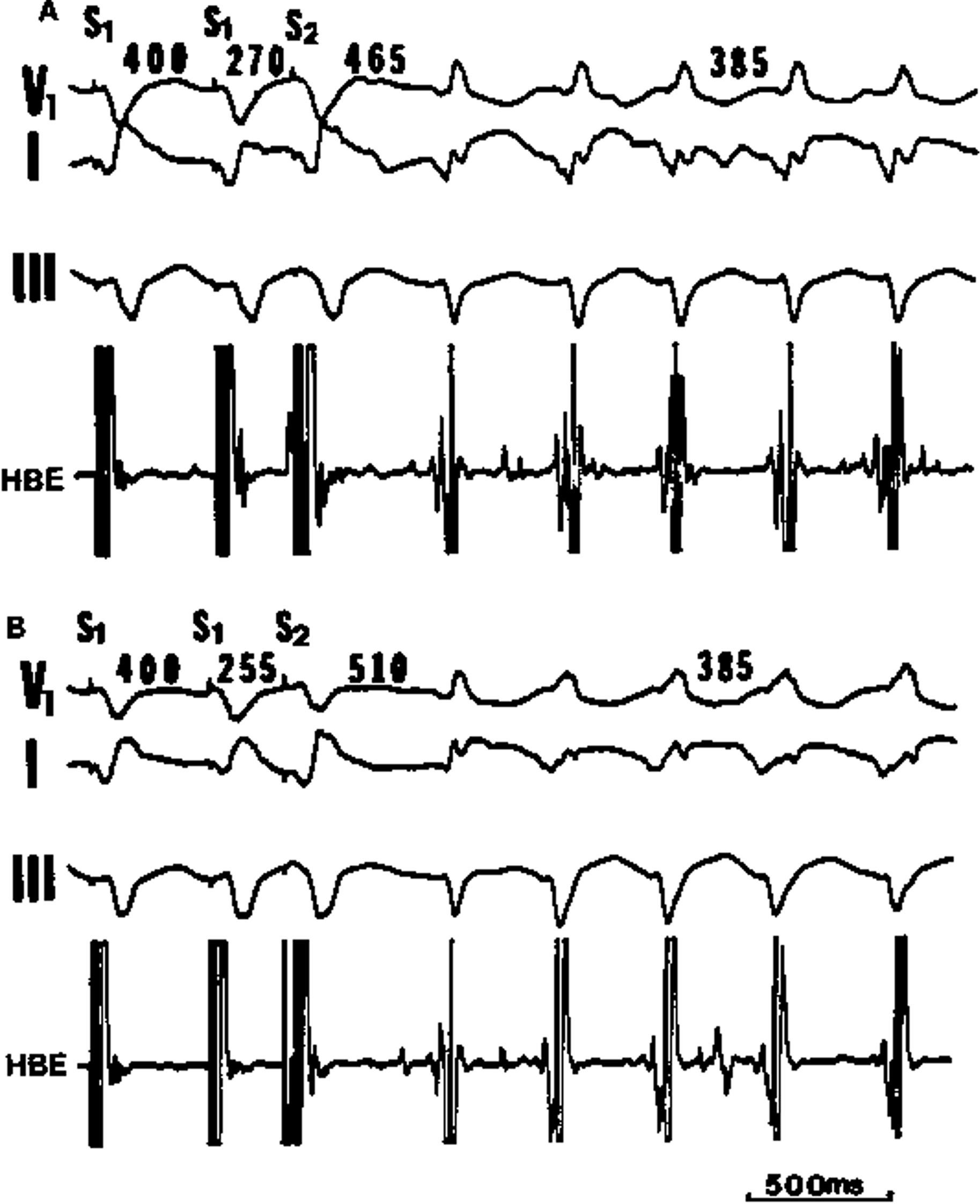
Initiation of ventricular tachycardia with programmed ventricular extrastimulation in a 59-year-old man with prior myocardial infarction associated with arteriosclerotic heart disease. During right ventricular pacing at a cycle length (SI-SI) of 400 ms, a ventricular premature beat (S2) with a premature coupling interval (SIS2) of 270 ms initiates an onset of ventricular tachycardia (VT) with a cycle length of 385 ms (A). Further shortening of the ventricular premature coupling interval (SI-S2) to 255 ms can also initiate the onset of VT (B). Note that the shorter the ventricular premature coupling interval (SI-S2), the longer the interval between the ventricular premature beat (S2) and the first beat of VT (465 vs. 510 ms). The VT can be terminated by overdrive ventricular pacing. These features suggest VT of a reentrant mechanism (Reference 37, Journal of Clinical Investigation with permission).
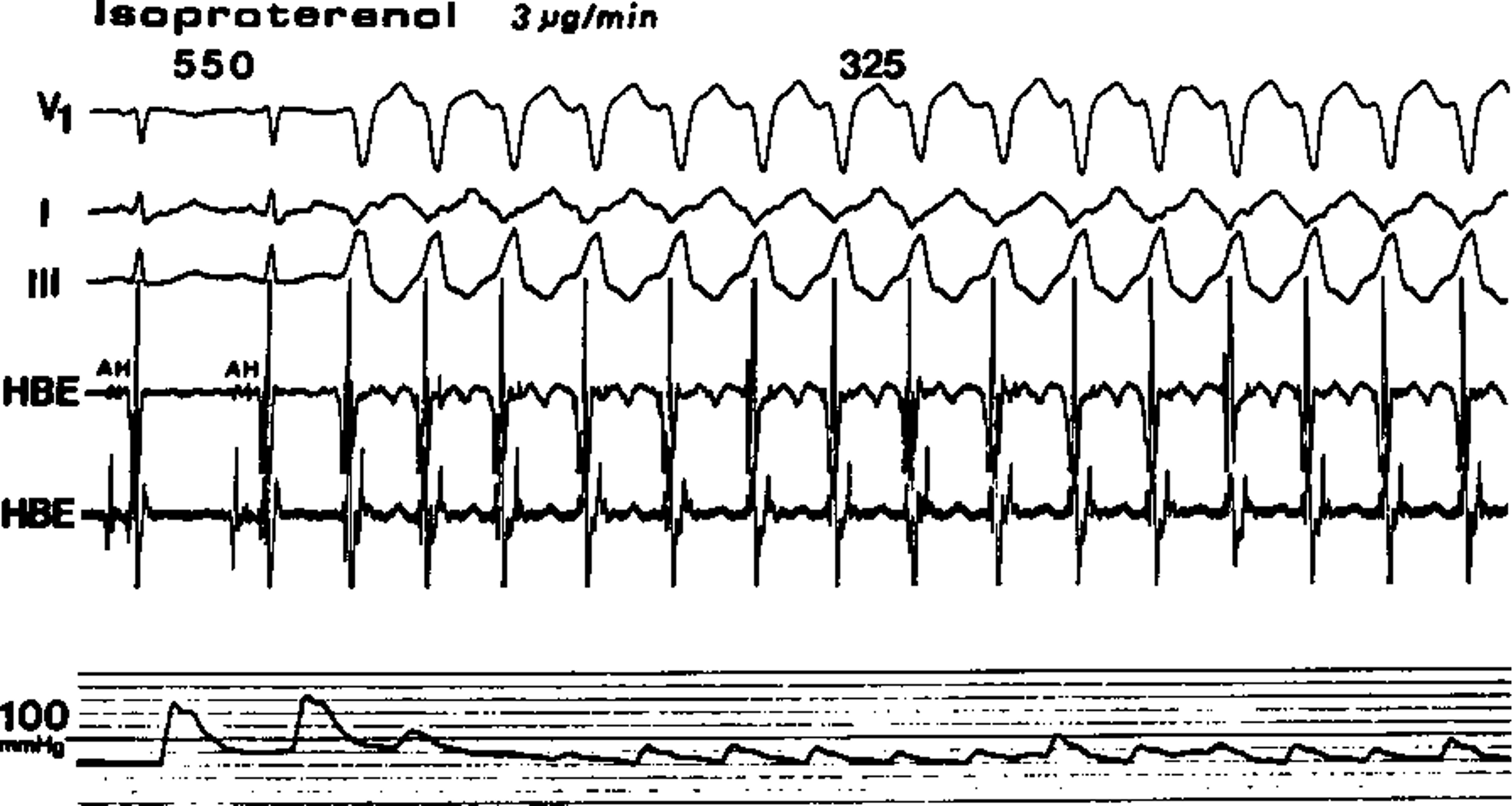
Induction of a paroxysm of ventricular tachycardia with intravenous infusion of isoproterenol in a 52-year-old man with no clinical evidence of organic heart disease. Following the failure of programmed electrical stimulation to induce arrhythmia, isoproterenol infusion at a dosage of 3 ug/min accelerates the sinus rate from 75 to 109 beats/min (cycle length of 550 ms) and provokes an onset of ventricular tachycardia with a cycle length of 325 ms. Right femoral arterial pressure is recorded at the bottom. The VT cannot be terminated by overdrive ventricular pacing. These features suggest the VT of an enhanced automaticity mechanism (Reference 37, Journal of Clinical Investigation with permission).
Cycle-length dependency for the initiation of ventricular tachycardia during sinus rhythm in an 18-year-old man with no clinical evidence of organic heart disease. At rest, the cycle length of sinus rhythm is 0.84 sec (A). The sinus rate then accelerates during handgrip. Ventricular tachycardia is initiated when the sinus rhythm attains cycle lengths between 0.53 and 0.49 sec (B and C). Further acceleration of the sinus rate (cycle length of 0.48 sec or shorter) initiates no ventricular tachycardia (D). Note that the interval between the first beat of ventricular tachycardia and its preceding sinus beat (QRS complex) shortens (0.52- 0.45 sec) as the sinus rate accelerates (B and C). During verapamil infusion, repeated handgrip accelerates the sinus rate with cycle lengths between 0.53 and 0.49 sec but fails to initiate ventricular tachycardia (E and F). These features suggest the VT of a triggered activity mechanism (Reference 37, Journal of Clinical Investigation with permission).
Induction of ventricular tachycardia (VT) during atrial-paced rhythms (same patient as above). (A) HRA pacing at a cycle length (S-S) of 660 ms (corresponding R-R interval, 660 ms) initiates no VT. (B, C, D) HRA pacing at cycle lengths (S-S) of 650, 570, and 470 ms, respectively (corresponding R-R intervals of 650, 570, and 500 ms, respectively) initiates an onset of VT. (E) HRA pacing at a cycle length (S-S) of 450 ms (corresponding R-R interval, 490 ms) initiates no VT. Note that the interval between the first beat of VT and its preceding atrial paced beat (QRS complex) gradually shortens as the atrial pacing rate progressively increases (B, C, and D). Following intravenous infusion of verapamil, atrial-paced rhythm can no longer induce VT. These features suggest the VT of a triggered activity mechanism (Reference 37, Journal of Clinical Investigation with permission). As discussed in Amendment on Verapamil-Sensitive Ventricular Tachycardia,” it is likely the VT has its first beat or few beats caused by triggered activity (manifested delayed after-depolarization [DAD] followed by the sustenance of reentrant VT due to altered substrate created by neighboring subthreshold unmanifested DADs.
The JCI is a broad medical journal, and it is ranked very highly in academic medicine. This accomplishment led to an invitation from Columbia University’s Dr. Andrew L. Wit and Dr. Michael R. Rosen’s Electrophysiology Research Group to deliver a special presentation at the American Heart Association annual meeting. In recognition of my achievements, UCSF promoted me to full professor on July 1, 1985. This was an exhilarating day for me.

In 1983, the University of Miami EP group reunion at the annual meeting of the American College of Cardiology. Benjamin Scherlag and Nabil El-Sherif (6th and 7th from left to right of the back row) and Madam Maria Castellanos, Ralph Lazarra, Tino Castellanos, and Robert Myerburg (1st, 2nd, 3rd, and 4th from left to right of the front row); Ming-Ren Young (first left of the back row standing next to Ruey Sung) and Sanjeev Saksena (first right of the back row).
Amendment on Verapamil-Sensitive Ventricular Tachycardia
Our research on verapamil-sensitive ventricular tachycardia (VT) revealed significant insights. Initially, we classified it as triggered activity, but further investigation showed it to have a reentrant mechanism. Our cellular simulation study demonstrated a crucial point: calcium overload not only generated triggered activity but also enriched the substrate’s properties, favoring reentry (Ref. 39). Subsequent simulation studies have confirmed this finding. These studies have shown that calcium overload generates delayed after-depolarizations (DADs), which can trigger activity once they reach the threshold. Meanwhile, the reduced sodium channel availability and increased gap junction coupling caused by subthreshold delayed afterdepolarizations (DADs) in neighboring regions, along with increased tissue heterogeneity, can lead to a unidirectional block of impulse transmission and thus promote the process of reentry (Reference 40,41). These findings suggest that verapamil-sensitive VT can be initiated by DAD-induced triggered activity, followed by a reentrant mechanism (reentrant VT). The reentry mechanism, still related to calcium overload, can be suppressed with verapamil. However, the mystery remains as to why this VT predominantly occurs in young individuals with structurally normal hearts. Future studies are needed to combine genetic analysis with functional studies of calcium handling to unravel this mystery.
Strikingly Different Triggers of Syncope Observed in Three Noteworthy Cases
Syncope, commonly known as fainting, often results from reduced blood flow to the brain. Heart rhythm disorders like tachycardia (fast heartbeat) or bradycardia (slow heartbeat) can cause syncope. However, as the following cases illustrate, the triggers of syncope can be surprisingly complex and diverse, adding an intriguing layer to our understanding of this condition.
The first case involved a 32-year-old business executive who experienced frequent fainting episodes nearly 20 times in just 6 months. Despite unremarkable physical examinations and a battery of negative tests, including exercise stress testing, 48-hour Holter monitoring (limited by the technology available at the time), and an invasive electrophysiological study with isoproterenol infusion, the cause remained elusive. Eventually, it came to light that these fainting episodes were triggered by psychological distress, specifically guilt or hysterical responses, stemming from his embezzlement of one million dollars from his company.
The second case involved a 22-year-old soldier who only fainted during rest periods following exercise. Interestingly, stress testing was able to replicate these symptoms during recovery from a high-intensity treadmill exercise protocol. ECG revealed sinus arrest with ventricular asystole as the underlying cause. Notably, this same phenomenon occurred in the electrophysiology (EP) lab when we stopped the challenge with isoproterenol infusion. Through comprehensive pharmacological testing with atropine and beta-blockers, along with repeated exercise tests, we concluded that the prolonged asystole resulted from an excessive parasympathetic rebound following the withdrawal of exercise-induced sympathetic activation (Ref. 42).
The third case involved a 68-year-old woman who experienced unexplained fainting episodes (three times in six months) without warning signs like palpitations or chest tightness. Unlike the previous cases, extensive testing wasn’t performed initially. Instead, an event recorder captured an episode of paroxysmal atrial fibrillation with a rapid ventricular rate (about 160/min). She received medication with a good response and eventually required a pacemaker due to sick sinus syndrome (tachycardia-bradycardia syndrome). This case emphasized the importance of considering various causes, including silent (asymptomatic) arrhythmias, in diagnosing syncope.
Investigating New Options for Arrhythmia Treatment
With the addition of Walter Reed Army Medical Center (WRAMC) as one of our research sites, we had a sufficient patient population to initiate a study on propafenone, a novel Vaughan-Williams class IC antiarrhythmic agent. This research launched the development of propafenone. Even today, propafenone remains a treatment of choice for atrial fibrillation (AF) and certain arrhythmias (such as supraventricular tachycardias, WPW syndrome, and ventricular arrhythmias) (Reference 43).
Subsequently, I led nationwide multicenter studies on two other promising drugs: esmolol and diltiazem. Esmolol, an ultra-short-acting β-blocker with a rapid onset of action (half-life of only 9 seconds), proved particularly beneficial for patients requiring temporary β-adrenergic blockade (Ref. 44); diltiazem, a calcium channel blocker (45), was similar to verapamil, belonging to Vaughan-Williams Class IV antiarrhythmics. We also studied nadolol, a long-acting β-blocker with a sustained effect (half-life of 15.5 hours) available in oral form (Refs. 46, 47), which offered a suitable option for long-term management when appropriate.
These studies, conducted in collaboration with excellent clinical researchers, provided valuable insights into the efficacy and safety of these novel drugs. Working with these esteemed colleagues was a genuinely significant experience for me.
A Shift in the Perspective Regarding Patient Care
One morning, a visit from a patient’s wife delivered a powerful message. She calmly handed me a letter written by her husband before his passing. His words moved me deeply, expressing gratitude for my medical expertise while also revealing his despair upon learning the grim prognosis of his end-stage heart disease. A wave of sadness washed over me as I realized the impact of my communication. Looking back, I acknowledged that my focus on scientific facts may have inadvertently excluded a vital component of care: “Encouragement with Hope.”
For the following week, I struggled with a lack of appetite, consumed by regret; this pivotal moment prompted me to reevaluate my approach to patient care. Could I offer something more than the traditional “five-finger approach” of medical history, physical exam, ECG, chest X-rays, and lab tests? I came to understand that the answer was a resounding yes. By cultivating patients’ hope through optimism and encouragement, I could improve patient outcomes, reduce hospitalization rates, and even extend lifespans.
Beyond Academia: Community Engagement and International Collaboration
While at UCSF, I dedicated my time to more than academia. During my leisure hours, I actively engaged with the Taiwanese American community, including founding the Taiwanese American Patriots Association—Peninsula Section (中半島台灣同鄉會) and the Northern California Taiwanese Physicians Association (北加州台灣醫師協會). I also regularly participated in activities at Canaan Taiwanese Christian Church (迦南台灣基督教會). I often shared these endeavors and experiences with Shin Wu (吳新一), my high school classmate who assisted with planning and execution.
My expertise extends beyond the borders of the United States. I have been invited to give lectures and conduct electrophysiology research at several academic medical centers in Taiwan and China, including Taipei Veterans General Hospital, my alma mater, National Taiwan University College of Medicine, Shanghai Medical College, and Peking University. This international exchange has fostered collaboration, and during my tenure at the University of California, San Francisco, I have also welcomed several trainees from Taiwan and China. One of these who deserves special mention is Dr. Wen-Der Lai (賴文德) from Kaohsiung Medical University (高雄醫學大學 [KMU]) in Taiwan. He worked with me at UCSF for one and a half years. After returning to Taiwan, we continued to collaborate on research projects, culminating in co-authoring several papers (Ref. 30,31,34,48-50). Among them, a paper on the physiology of dual AV nodal pathway conduction was published in the journal Circulation (Ref. 49), in which we clearly demonstrated the presence of an excitable gap in slow-fast AVNRT. KMU recognized his accomplishments and promoted him to full professor within three years. He later became the superintendent of the Kaohsiung Medical University affiliated Hospital (高雄醫學大學附設中和紀念醫院).
A Father’s Illness and a Doctor’s Helplessness
In 1987, my parents visited me in San Francisco for the first time in nearly a decade. Their arrival was joyous, but a troubling development quickly overshadowed it. Throughout their stay, Dad exhibited unusual thirst and weakness, symptoms he’d been experiencing back home in Taiwan. Having no history of diabetes in our family, I was alarmed. A visit to Moffitt-Long Hospital confirmed my fears – Dad’s blood sugar levels were alarmingly high, and further tests revealed a pancreatic head tumor. The news was a sudden and devastating shock.
Pancreatic cancer, even then, was a formidable foe, and at 69, Dad seemed particularly vulnerable. Consulting with specialists, including those in Boston, only solidified the grim prognosis. Surgery, the Whipple procedure, offered a slim chance of survival, and Dad was understandably hesitant. The limited treatment options left me feeling utterly helpless, a feeling I had never experienced as a physician. The following weeks were a blur of hospital visits and a gradual worsening of Dad’s condition. Jaundice set in, a telltale sign of a blocked bile duct, further complicating his situation. UC San Francisco facilitated a procedure to alleviate the blockage, but it was a temporary solution.
Watching my once vibrant father deteriorate was a heart-wrenching experience. The guilt of not being able to help him as a doctor added to the emotional burden. Sleep became a luxury, my appetite vanished, and my usual optimism faded. I turned to alternative medicine, seeking hope from Chinese herbalists. Their sincerity was evident, but the results were disappointing. My visits to Taiwan became more frequent, a constant back-and-forth between San Francisco and home. Thankfully, the pain remained elusive for most of his illness. However, after a period of in-and-out hospital stays, Dad passed away peacefully.
Over three decades have passed, and the fight against pancreatic cancer continues. Early diagnosis is paramount, and advancements have been made in identifying pre-cancerous conditions. Yet, the question of who should undergo preventive endoscopic imaging remains a puzzle. But the fight continues, and with each discovery, we move closer to a solution. This experience profoundly impacted me, highlighting the limitations of medicine and the immense emotional strain placed on families facing such a formidable illness. The toll it took on our family was immeasurable, and it’s a burden many families facing similar situations can relate to.
A Chance Encounter and a Life-Changing Decision
In 1990, clinical cardiac electrophysiology, once a field of theoretical exploration, became a powerful clinical tool. The technique of catheter ablation, using radiofrequency energy to target arrhythmias, revolutionized treatment for conditions like WPW syndrome. I heard that Stanford was searching for a leader to build a comprehensive electrophysiology program, and I strongly felt that I would like to be a part of it. Getting this position is no sure thing – competition can be fierce with many highly qualified candidates vying for the role. But I submitted my application, and Stanford, impressed by my academic record, invited me to deliver a lecture and a chance to outline a five-year vision for the program.
A significant concern for Stanford was securing a steady flow of patients. Over my nine years at UCSF, I cultivated strong relationships with physicians across the Bay Area, many of whom are UCSF alums. Drawing on the experience of building an EP group with VAH and LAMC, I reached out to Dr. Charles Young of Kaiser Permanente, whom I had never met before. Kaiser Permanente was an integrated managed care consortium and owned many local hospitals in the San Francisco Bay area. Young, too, harbored ambitions for an EP program at Kaiser but faced similar challenges. Over lunch, a plan hatched. Stanford could become a referral center for Kaiser, fostering the growth of both programs. As we shared a common vision, we anticipated everlasting camaraderie. I laid out this network of potential referral sources in my five-year plan, including Kaiser Hospitals and Dr. Young.
During my interview, the chair of the search committee, Professor Richard Popp, seemed genuinely impressed with my plan and recent publications in the prestigious Journal of Clinical Investigation (JCI). My lecture, delivered to Stanford’s cardiology department, went smoothly. However, a fellow Taiwanese researcher pulled me aside with a stark warning. Stanford’s cardiology department, he said, “Here is a mess right now, like Beirut in Lebanon.” His words gave me pause, but a spark of defiance ignited me. “I know the situation,” I replied, “and that’s precisely why it’s the perfect time to join. Things can only get better from here.” He seemed taken aback by my relentless optimism, but I knew that it was this optimism that would inspire change and progress.
Later, I learned the final decision was between me, a clinical cardiac electrophysiologist, and Dr. Gerry Griffin, a cardiac pacemaker specialist. When Stanford offered me the position, I was filled with a surreal but exhilarating sense of excitement. At the final negotiation, Stanford conferred me a tenured post as a professor of cardiovascular medicine for life. Years of struggle and dedication at UCSF’s San Francisco General Hospital (SFGH) had finally paid off. This wasn’t just a career move; it was the beginning of an exciting new chapter, a chance to be a part of something genuinely transformative.
References
(27). Shen EN, Sung RJ. Initiation of atrial fibrillation by spontaneous ventricular premature beats in concealed Wolff-Parkinson-White syndrome. American Heart Journal, 1982;103:911-912.
(28). Bhandari AK, Quock C, Sung RJ. Polymorphous ventricular tachycardia associated with a marked prolongation of the Q-T interval induced by amiodarone. Pacing and Clinical Electrophysiology, 1984;7:341-344.
(29). Tai DY, Chang MS, Svinarich JT, Chiang BN, Sung RJ. Mechanisms of verapamil-induced conduction block in anomalous atrioventricular bypass tract. Journal of the American College of Cardiology, 1985;5:311-317.
(30). Huycke EC, Nguyen NX, Lai WT, Sung RJ. Spontaneous conversion of atrial flutter to antidromic atrioventricular reciprocating tachycardia in the Wolff-Parkinson-White syndrome. American Heart Journal, 1988;115:917-919.
(31). Lai WT, Huycke EC, Keung EC, Nguyen NX, Tseng CD, Sung RJ. Electrophysiological manifestations of the excitable gap of orthodromic atrioventricular reciprocating tachycardia as demonstrated by single extrastimulation. American Journal of Cardiology, 1989;63:545-555.
(32). DiCarlo LA, Morady FI, Schwartz AB, Shen EN, Baerman JM, Krol RB, Scheinman MM, Sung RJ. Clinical significance of ventricular fibrillation-flutter induced by ventricular programmed stimulation. American Heart Journal, 1985;109:959-963.
(33). Nguyen NX, Yang P, Huycke EC, Keung EC, Deedwania P, Sung RJ. Effects of beta-adrenergic stimulation on atrial latency and atrial vulnerability in patients with paroxysmal supraventricular tachycardia. American Journal of Cardiology, 1988;61:1031-1036.
(34) Huycke EC, Lai WT, Nguyen NX, Keung EC, Sung RJ. Role of intravenous isoproterenol in the electrophysiologic induction of atrioventricular node reentrant tachycardia in patients with dual atrioventricular node pathways. American Journal of Cardiology, 1989;64:1131-1137.
(35) Sung RJ, Shen EN, Morady F, Scheinman MM, Hess D, Botvinick EH. Electrophysiologic mechanism of exercise-induced sustained ventricular tachycardia. American Journal of Cardiology, 1983;51:525-530.
(36) Rosen MR, Reder RF. Does triggered activity have a role in the genesis of cardiac arrhythmias? Annals of Internal Medicin, 1981;94:794-801.
(37) Sung RJ, Shapiro WA, Shen EN, Morady F, Davis J. Effects of verapamil on ventricular tachycardias possibly caused by reentry, automaticity, and triggered activity. Journal of Clinical Investigation, 1983;72:350-60.
(38) Sung RJ, Keung EC, Nguyen NX, Huycke EC. Effects of beta-adrenergic blockade on verapamil-responsive and verapamil-irresponsive sustained ventricular tachycardias. Journal of Clinical Investigation, 1988;81:688-699.
(39) Sung RJ, Wu YH, Lai NH, Teng CH, Luo CH, Tien HC, Lo CP, Wu SN. Beta-adrenergic modulation of arrhythmogenesis and identification of targeted sites of antiarrhythmic therapy in Timothy (LQT8) syndrome: A theoretical study. American Journal of Physiology-Heart and Circulatory Physiology , 2010;298:H33-H44.
(40) Liu MB, de Lange E, Garfinkel A, Weiss JN, Qu Z. Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm. 2015;12:2115-24.
(41) Scacchi S, Pavarino LF, Mazzanti A, Trancuccio A, Priori SG, Colli Franzone P. Transmural APD heterogeneity determines ventricular arrhythmogenesis in LQT8 syndrome: Insights from Bidomain computational modeling. PLoS One. 2024;19(7):e0305248.
(42) Huycke EC, Card HG, Sobol SM, Nguyen NX, Sung RJ. Postexertional cardiac asystole in a young man without organic heart disease. Annals of Internal Medicine, 1987;106:844-5.
(43) Shen EN, Keung E, Huycke E, Dohrmann ML, Nguyen N, Morady F, Sung RJ. Intravenous propafenone for termination of reentrant supraventricular tachycardia. A placebo-controlled, randomized, double-blind, crossover study. Annals of Internal Medicine, 1986;105:655-61.
(44) Sung RJ, Blanski L, Kirshenbaum J, MacCosbe P, Turlapaty P, Laddu AR. Clinical experience with esmolol, a short-acting beta-adrenergic blocker in cardiac arrhythmias and myocardial ischemia. Journal of Clinical Pharmacology, 1986;26:A15-A26.
(45) Huycke EC, Sung RJ, Dias VC, Milstein S, Hariman RJ, Platia EV and the Multicenter Diltiazem PSVT Study Group. Intravenous diltiazem for termination of reentrant supraventricular tachycardia: A placebo-controlled, randomized, double-blind, multicenter study. Journal of the American College of Cardiology, 1989;13:538-544.
(46) Chang MS, Sung RJ, Tai TY, Lin SL, Liu PH, Chiang BW. Nadolol and supraventricular tachycardia. Journal of American College of Cardiology, 1983;2:894-903.
(47) Sung RJ, Olukoton AY, Baird CL, Huycke EC, The Nadolol Research Group. Efficacy and safety of oral nadolol for exercise-induced ventricular arrhythmias. American Journal of Cardiology, 1987;60:15D-20D.
(48) Lai WT, Wu SN, Shen SH, Hwang YS, Sung RJ. Negative dromotropism of adenosine under beta-adrenergic stimulation with isoproterenol. American Journal of Cardiology, 1992;70:1427-1431.
(49) Lai WT, Lee CS, Sheu SH, Hwang YS, Sung RJ. Electrophysiological manifestations of the excitable gap of slow-fast AV nodal reentrant tachycardia demonstrated by single extrastimulation. Circulation. 1995;92:66-76.
(50) Sung RJ, Kuo CT, Wu SN, Lai WT, Luqman N, Chan NY. Sudden Cardiac Death Syndrome: Age, Gender, Ethnicity, and Genetics. Acta Cardiologica Sinica, 2008;24:65-74.
Stanford University (March 1, 1991 - June 30, 2001)
Establishment of the Cardiac Electrophysiology and Arrhythmia Service (CEAS)
Upon my arrival at Stanford University Medical Center (SUMC), I took the initiative to establish the Cardiac Electrophysiology and Arrhythmia Service (CEAS). Our initial team, consisting of myself, Bing Liem, Michael Lauer (my first EP fellow at Stanford) (later joined by Sung Chun), and nurse Jane Peterson (later joined by Linda Ottoboni and Michelle Gould), faced the challenge of competing with the University of California, San Francisco (UCSF) for patient referrals and research resources. However, our dedication and hard work paid off, as news of our arrival at SUMC sparked a surge in patient referrals, quickly reaching 3-4 times the previous volume and continuing to increase. To accommodate this growth, we extended our work hours until 7-9 pm. Most patients required catheter ablation for atrioventricular reciprocating tachycardia (AVRT [Wolff-Parkinson-White syndrome]), atrioventricular nodal reentrant tachycardia (AVNRT), atrial flutter, and ventricular tachycardia, and implantation of pacemakers and implantable cardioverter/defibrillators (ICDs). For patients referred from Kaiser Permanente, Charlie Young would often join us for EP studies and related procedures, highlighting the collaborative nature of our work. Our team’s collaboration was instrumental in the growth and success of the CEAS. This was a testament to the power of teamwork in achieving our goals.
To alleviate work pressure, every Friday afternoon after 5 pm, our team would gather at a restaurant in the Stanford Shopping Center to enjoy snacks, drinks, and camaraderie. We called it “TGIF” (Thank God It’s Friday). Through these experiences over the years, our team honed our skills in intracardiac mapping, radiofrequency catheter ablation, device implantation, and, later, laser-assisted lead extraction for cardiovascular implantable electronic devices. As expected, the demand for ICD implantation also increased significantly, reaching 100-120 cases per year. To accommodate this growth, SUMC renovated two dedicated spaces for us (SUMC has a total of 10 catheterization labs for all departments to use). Room 2 focused on pacemaker and ICD implantation, while we used Room 5 for EP studies and radiofrequency catheter ablation. Room 5 holds a special place in my heart, equipped with surround sound (music used to soothe patients), which I later donated to SUMC in honor of my father, Mr. Sen-Turng Sung (宋森滕).
Dr. Mark Anderson performed catheter ablation of arrhythmia in Room No. 5.
Outreach Program: Expanding Our Impact in Cardiac Arrhythmia Care
I initiated an Outreach Program to expand our reach and impact in cardiac arrhythmia care. We provided consultation services to neighboring hospitals, including San Jose Hospital, O’Connor Hospital, El Camino Hospital, and Washington Hospital. Additionally, I actively gave lectures in the San Francisco Bay Area, Southern California, and other states (such as Oregon and Nevada) to share insights on arrhythmia mechanisms and treatment options, raising awareness among general practitioners about arrhythmia management.
Furthermore, we organized an annual symposium on cardiac arrhythmias (Stanford Annual Cardiac Arrhythmia Symposium) that significantly contributed to the professional development of participating physicians, providing them with free CME (continuing medical education) credits. Lastly, we hosted an annual family gathering (ICD Support Group) for ICD patients (primarily sudden cardiac death survivors) and their families, providing education on daily care and medical knowledge. This event instilled optimism and hope that they could continue to live happy and meaningful lives. The symposium and the ICD Support Group were made possible through the generous sponsorship of device and pharmaceutical companies like Medtronic and St. Jude.
As our program expanded, we were delighted to welcome one additional fellow each year, sponsored by the Hong Kong Heart Association. This collaboration not only enhanced the global reach of our program but also provided valuable opportunities for international exchange and learning. Through SUMC, I also facilitated the licensure for trainees from Hong Kong, enabling them to rotate between training at SUMC and Kaiser Permanente medical facilities. This collaboration established Santa Clara Kaiser Hospital as a teaching hospital for clinical electrophysiology fellowship training. Naturally, Charlie Young was the Director of the Kaiser’s training program.
It is worth mentioning Professor Edward D. Harris, Jr., Chair of the Department of Medicine (1937-2010), and Professor Victor Dzau, Chair of Cardiovascular Medicine. They both provided immense support in the rapid establishment and development of my program – the CEAS division. Their vision and leadership were undoubtedly critical to our success.
Clinic Referrals and Personal Role Models
Stanford University Hospital (SUMC) is a private referral medical center, unlike the community hospital in San Francisco, San Francisco General Hospital (SFGH). Our team of doctors sees patients in the outpatient clinic two to three mornings a week. These patients are referred by doctors from different regions (including out-of-state); as such, we occasionally encounter some celebrities. The two professors I remember most vividly, because of their contrasting personalities, left a memorable impression on me.
Arthur Kornberg (1918-2007) was a distinguished professor at Stanford University and a Nobel laureate in Physiology or Medicine in 1959. He was not just a colleague but a friend. His discovery of DNA polymerase, the enzyme responsible for DNA replication, revolutionized our understanding of life. This enzyme enabled scientists to create copies of DNA, the molecule that carries genetic information in all living organisms. His research paved the way for the development of new drugs and technologies to combat diseases such as cancer and AIDS. Our professional interactions evolved into a sincere friendship, and he even sought my medical advice. This personal connection with such a great mind had a profound influence on my life. Professor Arthur Kornberg was undoubtedly a role model in my life.
In stark contrast was Edward Teller (1908-2003), an emeritus professor at the University of California at Berkeley, often referred to as the ‘Father of the Hydrogen Bomb.’ His contributions to nuclear physics, molecular physics, spectroscopy, and surface physics were not just undeniable, they were monumental. His work commanded the utmost respect, and his influence on the field of science was profound. Watching the movie ‘’Oppenheimer,” I was surprised to discover his involvement in creating the atomic bomb that ended World War II. The film also explored the moral dilemmas surrounding the development of the hydrogen bomb and the profound impact it had on people’s lives. Whatever the case, Professor Edward Teller was, in my eyes, a seasoned and respected elder.
Thriving Fields
As catheter ablation became a method for treating arrhythmias, I was frequently invited to present our team’s work at national and international conferences. These presentations focused primarily on the electrophysiology of the atrioventricular node (AV node) and AVNRT ablation techniques. I consistently advocated that selective ablation of slow pathway conduction is the most logical approach to eradicating this arrhythmia (Ref. 25). Our team actively conducted research in this area and published several articles on the subject (Refs. 51-57).
In addition, we spearheaded a multicenter study on the novel antiarrhythmic drug sotalol. Sotalol belongs to Vaughan Williams class III drugs but also has some beta-adrenergic receptor blocking effect (Ref. 58). Furthermore, to strengthen the clinical project, I asked Sung Chen to learn the technique of laser-assisted lead extraction and later urged Charlie Young to advance the catheter ablation of atrial fibrillation project.
We have had experience with amiodarone since the mid-1970s (Ref. 59). A national research team invited us to participate in the AICD (Antiarrhythmics Versus Implantable Defibrillators) study, comparing the efficacy of ICDs and oral amiodarone in preventing sudden death (Ref. 60). I actually felt that the comparison was illogical and unfair, as amiodarone was used to suppress the occurrence of VT/VF. In contrast, ICDs were used to terminate them once they occur. Therefore, the AICD study was comparing apples to oranges.
Nevertheless, this research path led me to explore the concept of ventricular vulnerability in these patients. At the time, determining the ventricular defibrillation threshold (DFT) involved using low-energy shocks (0.1 and later 0.2 joules) (Ref. 61). This approach allowed me to observe the “ventricular vulnerability period (VVP)” (Ref. 62). In the supine position, patients typically lose consciousness within 8 seconds after induction of ventricular fibrillation (VF). We measured that the VVP exists from the ascending limb of the ECG T wave to the peak of the T wave or slightly beyond the T wave peak, accounting for 12.2 +/- 5.8% of the QT interval. We used the midpoint of the VVP to determine the ventricular fibrillation threshold (VFT). This VVP midpoint fell between 0 and 90 milliseconds (mean 32.9 +/- 26.0 msec) before the T wave peak. In 21 patients, 16 (76.2%) had a VFT ≤ 0.2 joules (approximately 57 volts), 3 had 0.4 joules (approximately 81 volts), and 2 had 0.6 joules (approximately 99 volts). Therefore, in general, the VF threshold was very low (≤ 0.2 joules).
In real life, we occasionally see someone fall immediately after being hit in the chest, a phenomenon known as “commotio cordis”. The existence of VVP can explain this phenomenon. I was pleased that through this research, I was able to clearly see for the first time that we could more accurately predict the duration of VVP from the ECG. Later, I also learned about the dynamic nature of VVP, VFT, and DFT, which could be affected by changes in heart rate (cycle length), ischemia, different drugs, electrolyte imbalances, etc., all of which were related to the mechanism of sudden cardiac death.
Basic Electrophysiology Laboratories
Stanford University was very generous. In addition to two clinical electrophysiology laboratories, I was also given two basic science electrophysiology research laboratories, one for microelectrode recording and one for animal experiments. Although I had conducted feline animal experiments with Arthur Bassett at the University of Miami, I invited Stanford University colleague Associate Professor William Clusin and researchers from the Netherlands, Hanno Tan, and from China, You-Wen Qian, to establish a blood-perfused rabbit heart model in the animal laboratory. As for the microelectrode recording research laboratory, fortunately, Edmond Keung from the University of California, San Francisco (UCSF) volunteered to mentor Tong Lu, a researcher from China. Subsequently, Shien-Fong Lin, an optical imaging expert from Vanderbilt University, joined our rabbit experiments.
Our pursuit of basic science research was fruitful. We developed a new technique, selective coronary artery branch occlusion (Ref. 63), which could be used for the ablation of arrhythmias. This latter technique had the potential to significantly advance the field of electrophysiology by providing a more effective method for treating arrhythmias. In the blood-perfused rabbit heart, we could study the mechanisms of QT prolongation and the occurrence of torsade de pointes (Ref. 64). We found that activation of adenosine A1 receptors through adenosine-sensitive potassium channels could prevent torsade de pointes (Ref. 65). Additionally, we found that KN-93 (a multifunctional Ca++/calmodulin-dependent protein kinase inhibitor) could decrease early afterdepolarizations in rabbit heart (Ref. 66). Multifunctional Ca++/calmodulin-dependent protein kinase was later shown to play an essential role in arrhythmia occurrence (Ref. 67). Anderson, Schulman, and I were granted a patent for “Treatment of arrhythmias using Ca++/calm odulin-dependent protein kinase inhibitors.”
Finally, using advanced optical imaging techniques in blood-perfused rabbit hearts, we demonstrated a clear link between calcium transient alternans and its spatial heterogeneity in the early stages of myocardial ischemia and action potential alternans (Refs. 68, 69). These findings provided crucial insights into the underlying mechanisms of electrocardiogram ST-segment alternans (with or without concomitant QRS complex alternans) observed in clinical settings of acute myocardial ischemia. To paraphrase, in the clinical setting of acute myocardial ischemia, the development of ST-segment elevation alternans could serve as a warning sign for impending serious ventricular arrhythmias, such as ventricular fibrillation.
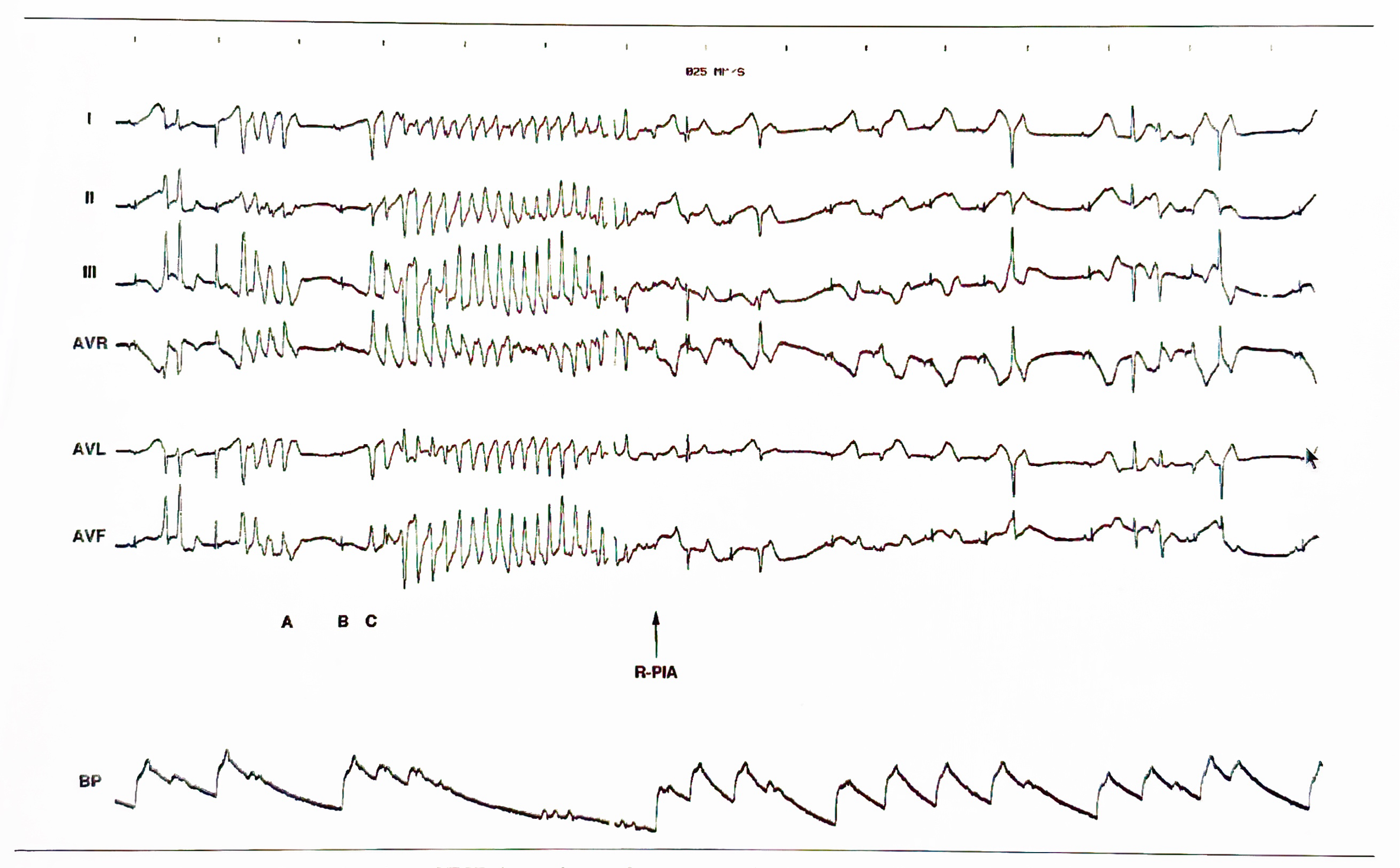
Mode of onset and termination of TdP (torsade de pointes) in the blood-perfused rabbit heart. From top to bottom: standard electrocardiographic limb leads (I, II, III, AVR, AVL, AVF) and arterial blood pressure (BP). An episode of TdP, which is characterized by an apparent twisting of the mean electrical axis within each electrocardiographic lead around the isoelectric line and a drop of arterial blood pressure, starts with a ‘short-long-short’ sequence of RR coupling intervals: a PVC (premature ventricular complex) with a short coupling interval (A) (in this case: the last PVC in a series of coupled PVCs) is followed by a sinus beat with a long coupling interval (B), which is followed, after a short coupling interval, by a ventricular complex (C) which constitutes the first beat of TdP. Immediately after TdP onset, methoxamine/clofilium infusion is stopped, and R-PIA (R-phenylisopropyladenosine), a metabolically stable adenosine A1 receptor agonist, is infused (arrow). Within seconds, PVC incidence decreases, and sinus rhythm is eventually restored (Reference 65, Cardiovascular Drugs and Therapy with permission).
A, Recording of [Ca2+]i (Calcium transient) alternans during ischemia in a blood-perfused heart. Alternans ratio is obtained as 1−B/A, where B is the net amplitude of the smaller transients, and A is the net amplitude of the larger transients. B, Calcium transients from 2 pixels in a blood-perfused ischemic heart showing alternans of the same phase. The heart image gives pixel location. Regions of the image appearing black are outside the heart margin or dimly illuminated. Pixel coordinates are (0,0) at the top left and (96,96) at the bottom right. Each tracing represents 1 second. C, Calcium transients from two pixels showing alternans that are out of phase. Same heart and image files as panel B (Reference 68, Circulation with permission).
Stanford’s Global Impact and Personal Milestones
Stanford Cardiovascular Medicine has consistently ranked among the best in the world and played a significant role in fostering international relationships. This has been evident in the diverse patient population it attracts, with referrals coming from across the United States and internationally, particularly from Asia (Hong Kong, Singapore) and the Middle East (Saudi Arabia). This global reputation has naturally attracted charitable support from these regions, further strengthening Stanford’s influence on a worldwide scale.
Professor Norman E. Shumway (1923-2006), a pioneering heart transplant surgeon, and his protégé Dr. Bruce A. Reitz (Chief of Cardiovascular Surgery) performed the first successful heart and heart-lung transplant in the United States at Stanford. Alongside them were other cardiovascular surgery luminaries such as D. Craig Miller (mitral valve replacement), Philip E. Oyer (general cardiovascular surgery), Victor B. Dzau (hypertension, Chief of Cardiology), Richard L. Stinson (surgical treatment of arrhythmias), Richard J. Popp (echocardiography), Steve Oesterle (coronary angioplasty and stenting), John S. Schroder (heart failure), and Michael B. Fowler (heart failure).
Occasionally, the Stanford team would embark on overseas visits to strengthen ties with international donors and encourage patient referrals. I participated in two trips as the Director of Stanford CEAS (clinical cardiac electrophysiologist), visiting Taiwan and Hong Kong. In Taiwan, I facilitated a valuable meeting between the Stanford team and Professor Professors Shuxun Zhu (朱樹勳) and Tsun Wei (魏崢), (at the time the most prominent heart transplant surgeons in Taiwan). These trips were not solely focusing on work; they also provided opportunities for me to connect with loved ones. I cherished the moments of reuniting with my mother, friends, and relatives. Stanford’s distinguished reputation also served as the backdrop for some significant personal milestones, marking my growth and fulfillment in the field of cardiovascular medicine.

A, Recording of [Ca2+]i (Calcium transient) alternans during ischemia in a blood-perfused heart. Alternans ratio is obtained as 1−B/A, where B is the net amplitude of the smaller transients, and A is the net amplitude of the larger transients. B, Calcium transients from 2 pixels in a blood-perfused ischemic heart showing alternans of the same phase. The heart image gives pixel location. Regions of the image appearing black are outside the heart margin or dimly illuminated. Pixel coordinates are (0,0) at the top left and (96,96) at the bottom right. Each tracing represents 1 second. C, Calcium transients from two pixels showing alternans that are out of phase. Same heart and image files as panel B (Reference 68, Circulation with permission).

The Third International Symposium on Cardiac Arrhythmias, Taipei, Taiwan, May 4, 1996. Heins Wellens、Fred Morady、Wen-Pin Lien, Ruey Sung(the 3rd, 4th, 5th, and 6th from left in the front row)、Delon Wu(the 5th from the left in the back row)、Wen-Der Lai(the first right in the back row ) .
In 1995, I was delighted to host a welcome dinner for visiting scholar Academician Professor Chun Won Lee (李鎮源院士) at the Stanford Faculty Club. Continuing this tradition of fostering connections, I organized the annual meeting of the North American NTU Medical School Alumni Association (北美台大醫學院校友會) at the Marriott Hotel in 1998. The event spanned two nights and three days. Several esteemed guests from Taiwan graced the occasion, including Academician Chun Won Lee (李鎮源院士), Academician Rui Lou Song (宋瑞樓院士), Lee Yuan-Tseh (李遠哲Nobel Laureate諾貝爾獎桂冠, President of the Academia Sinica中央研究院院長), Qingjiang Lin (林清江Minister of Education;教育部長), Chengwen Wu (吳成文Director of the National Institutes of Health,國衞院院長), Academician Mingzh Zhuane, (莊明哲院士), and Professor Huang Dafu (黃達夫教授).

From left to right: Academician Mingzh Zhuane, (莊明哲院士); Professor Huang Dafu (黃達夫院長, Hexin Hospital [和信醫院]), and Dean Ruey Sung.
Loss and Legacy
During my time at the University of California, San Francisco (UCSF) and Stanford University, the experience of losing loved ones profoundly impacted my life. At UCSF, I mourned the passing of my father. Later, at Stanford, I experienced the loss of my father-in-law, Yibi Zeng (曾以碧), who passed away from lung cancer at the age of 85 in Japan. These losses, though deeply painful, also revealed the resilience within me, inspiring me to carry on their legacies.
My father-in-law was a remarkable man. He earned his medical degree from Nippon University (日本大學) during the Japanese colonial period in Taiwan. After that, he returned to Taiwan to work as a family doctor, eventually immigrating to Kobe, Japan, in the 1970s. From the very beginning, his warmth and generosity extended to me. He and my mother-in-law, Mrs. Shuji Chen (陳淑姬), treated me with the utmost respect and affection. It was significant that due to my influence, my in-laws and several of my wife’s siblings had become Christians. When my mother-in-law entrusted me with delivering a eulogy at his funeral, it was both a heartfelt responsibility and a lifelong honor, and a testament to the trust and respect they had for me. It was a weighty responsibility but one I was humbled and honored to carry.
Regrettably, time continued its relentless march forward, and my mother-in-law suffered from dementia at the age of 80 and eventually succumbed to it at the age of 87 at a nursing home. I witnessed her progressive mental decline, a painful journey that left me feeling utterly helpless. The once vibrant and loving woman became unable to enjoy the happiness of family gatherings, and her apathetic face was a stark reminder of the cruel nature of her illness. It was another source of profound grief for me.
Cultivating and Nurturing Exceptional Researchers
My leadership of the Clinical Electrophysiology Services (CEAS) extended beyond a focus on research and clinical work. I found immense satisfaction in mentoring exceptional researchers and witnessing their remarkable achievements:
Dr. Michael R. Lauer (MD, PhD): An expert in cellular calcium metabolism, Dr. Lauer joined the Stanford faculty in 2000. He was an invaluable assistant in training clinical EP fellows (Ref. 51-56,61). We co-authored a comprehensive textbook, “Fundamental Approaches to the Management of Cardiac Arrhythmias 2000” (Ref. 25). He later transitioned to a leadership role in clinical cardiac electrophysiology at Kaiser Permanente, ensuring the smooth continuation of the collaboration between Stanford and Kaiser, alongside Charlie Young.
Dr. Tong Lu (Ph.D.): Dr. Lu is from China and was introduced to me by Dr. Edmond Keung at UCSF. His potential was evident, and I encouraged him to establish a microelectrode laboratory at Stanford (Ref. 66). His work at the Mayo Clinic’s animal lab focused on the relationship between cardiovascular ion channel dysfunction and clinical conditions such as diabetes, heart failure, and arrhythmias, has been impactful. He is currently a research professor at the Mayo Clinic School of Medicine.
Dr. Hanno L. Tan (MD, PhD): Hailing from the Netherlands, Dr. Tan, introduced by Professor Jose Jalife at the State University of New York, brought expertise in animal models. He helped us establish a rabbit heart model in our animal research laboratory to investigate the mechanisms of QT prolongation and torsades de pointes (Refs. 58,64,65). Today, he is a world authority on inherited ion channelopathy and serves as Professor of Cardiology at the Amsterdam University Medical Center, Netherlands.
Dr. Mark Anderson (MD, PhD): During his clinical EP fellowship, Dr. Anderson, along with his essential science mentor, Professor Howard Schulman, invited me to participate in research on the role of calmodulin-dependent protein kinase in arrhythmogenesis (Ref. 66). Dr. Anderson’s work led him to become the Cardiology Chief at the University of Iowa, later serving as Chair of the Department of Internal Medicine. His research solidified his position as a world expert on calmodulin-dependent protein kinase. His career trajectory continued to rise, culminating in his role as Dean of the Johns Hopkins University School of Medicine and currently as Dean of Medical Affairs at the University of Chicago.
These former clinical electrophysiology fellows, including Dr. Wen-Ter Lai (賴文德) from Taiwan trained at UCSF (Ref. 30,31,34,48-50), showcase the profound impact one can have in nurturing the next generation of academic physicians. Witnessing their contributions to cardiology, particularly cardiac electrophysiology, fills me with immense pride.
Sabbatical Leave and New Opportunity
After a decade of intense work at Stanford University, where I established the Stanford Clinical Electrophysiology Services (CEAS), I found myself grappling with burnout and a longing for a different rhythm. It was at this juncture that I became eligible for sabbatical leave, a possibility that had opened up after seven or more years of service. With no specific plans in mind, a fortuitous opportunity presented itself through a friend’s introduction.
A prominent Taiwanese enterprise, Cathay Group (國泰集團), expressed interest in my expertise and sought me to fill a dual role. On one hand, they were looking for a physician to oversee the medical care of the company’s founder’s father. On the other hand, they needed someone who could help elevate their affiliated hospital, Cathay General Hospital (國泰綜合醫院), to a government-accredited medical center (認可的醫學中心). This latter position was defined as the Chief Executive Officer (CEO) for Cathay Group’s medical affairs.
Fascinated by the potential for personal growth and professional progress, I looked deeper into the opportunity. After negotiations, we reached an agreement on a compensation package that I found acceptable. These terms, along with the chance to be closer to my family in Taiwan, aligned perfectly with my aspirations. I saw this as an unexpected opening and decided to use my Sabbatical Leave to embrace the challenge of this unique dual role.
References
(51) Young C, Lauer MR, Liem LB, Sung RJ. A characteristic electrocardiographic pattern indicative of manifest left-sided posterior septal/paraseptal accessory atrioventricular connections. American Journal of Cardiology, 1993;72:471-475.
(52) Lauer MR, Young C., Liem LB, Sung RJ. Efficacy of adenosine in terminating catecholamine dependent supraventricular tachycardia. American Journal of Cardiology, 1994;73:38-42.
(53) Yu JC, Lauer MR, Young C, Liem LB, Hou C, Sung RJ. Localization of the origin of the atrioventricular junctional rhythm induced during selective ablation of slow-pathway conduction in patients with atrioventricular node reentrant tachycardia. American Heart Journal, 1996;131:937-946.
(54) Lee KL, Lauer MR, Young C, Lai WT, Tai YT, Chun H, Liem LB, Sung RJ. Spectrum of electrophysiologic and electropharmacologic characteristics of verapamil-sensitive ventricular tachycardia in patients without structural heart disease. American Journal of Cardiology, 1996;77:967-973.
(55) Young C, Lauer MR, Liem LB, Chun H, Sung RJ. Demonstration of a posterior atrial input to the atrioventricular node during sustained anterograde slow pathway conduction. Journal of American College of Cardiology, 1998;31:1615-1621.
(56) Lee KL, Chun HM, Liem LB, Lauer MR, Young C, Sung RJ. Multiple atrioventricular nodal pathways in humans: electrophysiologic demonstration and characterization. Journal of Cardiovascular Electrophysiology, 1998;9:129-140.
(57) Lee KL, Chun HM, Liem LB, Sung RJ. Effects of adenosine and verapamil in catecholamine-induced accelerated atrioventricular junctional rhythm: insights into the underlying mechanism. Pacing and Clinical Electrophysiology, 1999;22:886-870.
(58) Sung RJ, Tan HL, Karagounis L, Hanyok JJ, Falk R, Platia E, Das G, Hardy SA. Intravenous sotalol for the termination of supraventricular tachycardia and atrial fibrillation and flutter: a multicenter, randomized, double-blind, placebo-controlled study. Sotalol Multicenter Study Group. American Heart Journal, 1995;129:739-748.
(59) Desai AD, Chun S, Sung RJ. The role of intravenous amiodarone in the management of cardiac arrhythmias. Annals of Internal Medicine, 1997;127:294-303.
(60) Hallstrom AP, Greene HL, Wyse DG, Zipes D, Epstein AE, Domanski MJ, Schron EB; AVID Investigators. Antiarrhythmics Versus Implantable Defibrillators (AVID)–rationale, design, and methods. American Journal of Cardiology, 1995;75:470-475.
(61) Lauer MR, Young C, Liem LB, Ottoboni L, Peterson J, Goold P, Sung RJ. Ventricular fibrillation induced by low-energy shocks from programmable implantable cardioverter-defibrillators (in patients with coronary artery disease). American Journal of Cardiology, 199473:559-563.
(62) Hou CJ, Chang-Sing P, Flynn E, Martinez L, Peterson J, Ottoboni LK, Liem LB, Sung RJ. Determination of ventricular vulnerable period and ventricular fibrillation threshold by use of T-wave shocks in patients undergoing implantation of cardioverter/defibrillators. Circulation. 1995;92:2558-2564.
(63) Hsia TY, Billingham M, Sung RJ. Intracoronary arterial occlusion: A novel technique potentially useful for ablation of cardiac arrhythmias. Journal of Interventional Cardiac Electrophysiology, 1997;1:7-14.
(64) Tan HL, Hou CJ, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the prolonged QT interval syndromes and torsade de pointes. Annals pf Internal Medicine, 1995;122:701-714.
(65) Tan HL, Hou CJ, Sung RJ. Effects of adenosine A1-receptor activation on torsade de pointes in rabbits. Cardiovascular Drugs and Therapy, 1999;13:441-447.
(66) Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early after depolarization in rabbit hearts. Journal of Pharmacology and Experimental Therapeutics, 1998;287:996-1006
(67) Bengel P, Dybkova N, Tirilomis P, Ahmad S, Hartmann N, A Mohamed B, Krekeler MC, Maurer W, Pabel S, Trum M, Mustroph J, Gummert J, Milting H, Wagner S, Ljubojevic-Holzer S, Toischer K, Maier LS, Hasenfuss G, Streckfuss-Bömeke K, Sossalla S. Detrimental proarrhythmogenic interaction of Ca2+/calmodulin-dependent protein kinase II and NaV1.8 in heart failure. Nature Communication, 2021;12:6586.
(68) Qian YW, Clusin WT, Lin SF, Han J, Sung RJ. Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood-perfused rabbit heart. Circulation, 2001;104:2082-2087.
(69) Qian YW, Sung RJ, Lin SF, Province R, Clusin WT. Spatial heterogeneity of action potential alternans during global ischemia in the rabbit hearts. American Journal of Physiology-Heart and Circulatory Physiology, 2003;285:H2722-H2733.
Cathay General Hospital (CGH) (July 1, 2000 - June 30, 2001
In 1977, Cathay Life Insurance (國泰人壽now a subsidiary of Cathay Financial Holdings [國泰金控]) established Cathay General Hospital (國泰綜合醫院 [CGH]), a 400-bed private hospital known for its comprehensive medical services. When I took over as CEO, they were in the process of obtaining “medical center (醫學中心)” status, an essential accreditation in Taiwan’s National Health Care System (台灣國民健康保險制度 [NHCS]).
Dual Responsibilities: Patient Care and Hospital Development
Initially, I was responsible for overseeing the medical and nursing care of President Tsai’s father. While important, my primary focus soon expanded to include achieving CGH’s status as a ‘medical center.’ This accreditation, granted by the Joint Commission of Taiwan Hospital Association (台灣醫院協會聯合委員會 [JCTHA]), was a significant milestone that we all worked tirelessly to achieve. It had a profound impact on the reimbursement rates a hospital received from the Bureau of Health (衛生署), now the Ministry of Health and Welfare (衛生福利部; [衛福部]).
After evaluating Chairman Tsai’s father’s condition, I convened a meeting of the attending physicians from the relevant specialties. The meeting facilitated a discussion to gather their insights. Together, we set the direction for improving his care, focusing on correcting anemia, controlling heart failure, and alleviating shortness of breath. We also took preventive measures to minimize the risk of secondary respiratory and urinary tract infections. As he was taking digoxin, we ensured that digitalis antibody (antibody against digoxin) was readily available to address any potential toxicity. Fortunately, Chairman Tsai’s father’s condition remained stable during my tenure as CEO. Sadly, he passed away five years later from complications of metastatic cancer.
Challenging but Rewarding Challenges
After understanding the requirements for JCTHA “Medical Center” accreditation, I met with the attending physicians, including some department heads, to discuss areas that needed improvement. Every Monday at 9:00 a.m., I reported to Chairman Tsai on his father’s medical progress and my assessment of how CGH could improve to meet JTCH’s “Medical Center” requirements.
Among them, Dr. Ko De-Heng (高德亨), the head of Immunology, was the most enthusiastic and insightful. I am very grateful for his valuable advice and dedication. We quickly became good friends after our first meeting. Sadly, he and his wife were later killed in a plane crash.
Strategic Growth: A Journey of Progress
To move the hospital toward becoming a “medical center,” we prioritized rapid upgrades in critical areas. These included the internal medicine and surgical intensive care units, the obstetrics and gynecology labor and delivery unit, and the social services department. In addition, we purchased an intracardiac mapping system (Endocardial Solution) for the cardiac catheterization laboratory for use in electrophysiology (EP) studies and catheter ablation of cardiac arrhythmias.
Realizing the importance of ‘academic research ‘ in achieving ‘medical center ‘ accreditation, we embarked on a collaborative journey. The nearing completion of the Cathay Sijhih Research Center (國泰汐止研究中心 [CSRC]) was a significant milestone, and we identified all the physicians with research potential, formed a dedicated team, and appointed a director. To keep up with the trend in genetics research, we recruited gene sequencing personnel trained at Academia Sinica (中央研究院). Simultaneously, we acquired the most advanced equipment to enhance our research capabilities further. In addition, I helped them develop the CSRC’s organizational terms, which stipulated that they would receive a fixed annual budget from CGH, operate independently under the direct supervision of the Cathay Group chairman, but not be subject to the hospital’s administrative management.
Leading by Example
My commitment to the team went beyond leadership. I established my clinic, seeing referrals and providing consultations one afternoon each week.
Once, the cardiac catheterization lab encountered difficulty in handling a complex Wolff-Parkinson-White syndrome case and requested my assistance after three hours of struggling. To their surprise, I completed the AV bypass tract ablation within 15 minutes. This anecdote spread throughout the hospital the next day, boosting their confidence in my ability as CEO.
Additional Prospects and Unexpected Obstacles
President Junyan Chang (張俊彥) of National Chiao Tung University (國立交通大學 [NCTU]) harbored an ambitious vision to establish a unique medical school – the College of Biotechnological Medicine (生物科技醫學院; CBTM), conferring dual MD, PhD degrees to students. He reached out to Mr. Tsai, Chairman of the Cathay Financial Holdings Group, CGH superintendent Kai-Mo Chen (陳楷模), and me to discuss a mutually beneficial plan. This plan would elevate CGH’s academic programs beyond the standards of a “medical center” and transform it into a medical institution comparable to a general university-affiliated hospital. Recognizing the potential for CGH’s further advancement, I encouraged Chairman Tsai to sign a memorandum of understanding (MOU) with President Junyan Chang (張俊彥). Chairman Tsai readily agreed and generously pledged to donate NT$50 billion to build a new hospital on the NCTU campus in memory of his father, a gesture that we sincerely appreciated. This donation would be synchronized with the development of NCTU’s CBTM.
Further demonstrating his trust, President Junyan Chang (張俊彥) appointed me as an NCTU Distinguished Professor and Chair of the CBTM Planning Committee. He even provided an office on campus. There, I quickly assembled a team of professors from diverse disciplines, each bringing their unique expertise to the table. We modeled our planning after Stanford University’s James H. Clark Center (Bio X Center) meeting every Tuesday morning, fostering a collaborative and interdisciplinary approach to the project. However, this ambitious plan and vision sparked unexpected friction in the academic world. National Taiwan University (國立台灣大學), National Tsing Hua University (國立清華大學), National Yang-Ming Medical University (國立陽明大學; NYMU), and Taipei Veterans General Hospital (台北榮民總醫院) all expressed concerns and opposition. They argued that NCTU’s expansion into the medical field would disrupt the established balance of academic excellence, particularly in the field of biotechnology. They were also concerned about the potential impact on their own biotechnology and biomedical medical programs (e.g., loss of personnel). Consequently, they collectively blocked all applications submitted to the Ministry of Education (MOE) and the Bureau of Health (BOH), effectively halting our plans.
After both the MOE and the BOH denied NCTU’s application to establish a Biotechnology College of Medicine, President Chang proposed a merger with NYMU. The NCTU University Council approved the proposal, but unfortunately, the NYMU University Council rejected it, citing concerns about the potential loss of their institutional identity and autonomy. However, a few years later, under President Chang’s leadership, they established a new College of Biotechnology (but not a medical school) in 2005 and invited me to be the first guest speaker.
Ironically, no one could have predicted that more than two decades later, the merger of National Chiao Tung University and National Yang-Ming University – “National Yang-Ming Chiao Tung University” (established in 2021) – would become a reality, marking a significant and unexpected turn of events.
Strengthening Clinical Expertise and an Unexpected Turn
To strengthen CGH’s cardiovascular medicine program, I had previously tried to recruit cardiothoracic surgeons such as Dr. Zong-Bor Tsai (蔡宗博) from Chung-Shan Medical University and Dr. You-Ren Yang (楊友任) from National Cheng Kung University College of Medicine (NCKUMC). Unexpectedly, Dr. Yang instead suggested that I become a candidate for the deanship of NCKUMC. Although intrigued, I reaffirmed my original plan to return to Stanford after completing my CGH mission.
From Accolades to Crossroads
In May 2001, Cathay General Hospital (CGH) finally passed the JCTHA accreditation and became a “medical center”. The JCTHA committee acknowledged our efforts, praising the renovation of essential facilities, the excellent organization of the CSRC, the presence of a state-of-the-art gene laboratory in the center, and the availability of an advanced intracardiac endocardial mapping system in the catheterization laboratory. However, the completion of this mission coincided with the possibility of a life-changing journey, as I had received notification from NCKUMC that I was the top candidate for the deanship and they were awaiting my final decision.
It was during a chance meeting that I first met Professor Huan-Yao Li (黎煥耀), Director of the Institute of Microbiology and Immunology at NCKUMC. He earnestly urged me to accept the deanship, emphasizing the urgent need for new leadership at NCKUMC. I made a memorable trip back to Stanford University to consult with Professor Judy Swain, Chair of Internal Medicine, who informed me that my more than 10 years of service had qualified me for emeritus status at Stanford. I then requested a meeting with Professor Kun-Ying Huang (黃崑巖), the founding president of NCKUMC. At a breakfast meeting, he presented me with a list of ten faculty members who might be willing to assist me in fulfilling my duties as Dean. The list conspicuously included Professor Huan-Yao Li (黎煥耀). This unexpected opportunity marked a pivotal turning point in my career trajectory.
National Cheng Kung University College of Medicine (July 1, 2001- August 31, 2007)
Upon assuming the role of Dean of the College of Medicine at National Cheng Kung University (NCKU), I presented my vision to the University President Chiang Gao (高強). He placed his trust in me by asking me to concurrently serve as the University Vice President for Medical Affairs (approval pending faculty vote) and Director of the NCKU Biotechnology Center, a role strategically aligned with the College’s goals.

Ruey Sung at the Dean’s Office.
Formation with a Dedicated Team to Shape the Future
Building on my experience at UCSF, Stanford, and the Cathay Group, I immediately began assembling a solid administrative team. I invited several faculty members from the founding Dean, Kunyan Huang’s (黃崑巖) initial list, offering them Vice Dean or Associate Dean positions, mirroring leadership structures in U.S. medical schools. NCKU regulations allowed only one Vice Dean, so I designated this position as Executive Vice Dean. Our dedicated team was comprised of exceptional individuals:
Secretaries: Biyu Zhou (周碧玉), Mei-ing Lee (李美頴) (and others)
Vice Deans: Executive: Mingzhe Tang (湯銘哲), Junzhong Wu (吳俊忠) (later)
Faculty Affairs: Lin Mingde (林銘德)
Research: Lei Huanyao (黎煥耀)
General Affairs: Yichian Lee (李益謙)
Student Affairs: Chi Tsen Lin (林啟禎), Chongjie Luo (羅崇傑)
Admissions and High School Promotion- Xiujuan Lin (林秀娟), Lien-I Hor (何漣漪) (later)
Curriculum: Chihe Lin (林其和)
International Affairs: Huizhen Su (蘇慧貞)
Fundraising: Baolin Guo (郭保麟)
Together, we pursued a three-pronged mission: education (teaching), research (learning), and medical care (serving). Our guiding principle was, in addition to the core curriculum, the inseparable link between humanistic literacy and medical ethics. We championed a robust general and liberal arts education in the pre-med years and integrated medical humanities into daily life. Our vision was to cultivate well-rounded medical professionals. These professionals would possess exceptional medical skills, a solid moral compass, and a lifelong commitment to serving society. In short, we aimed to develop individuals who embodied both technical proficiency and virtue (術德兼備). The goal meant that students wouldn’t just acquire knowledge and skills; they would also learn the core values that guide a meaningful life.

“I will practice my profession with conscience and dignity; the health of my patients will be my first consideration.”- A physician’s oath.
We held a breakfast meeting every Wednesday morning at 7:30. One of my initial top priorities was to establish clear administrative guidelines, particularly regarding the definition of “National Cheng Kung University Medical Center.” The concept included the structure of the administrative and academic integrity of the medical school and affiliated hospital. Its roots sprung from the existing fact that the medical school had the authority to grant academic titles and approve essential documents of the affiliated hospital. The latter responsibility included the appointment of the affiliated hospital superintendent and the appointment of the Chiefs of Departments of Internal Medicine and Surgery.

National Cheng Kung University Medical Center.
Cultivating a Flourishing Academic Environment
Spearheaded by Vice Dean Yichian Lee (李益謙), we embarked on a comprehensive beautification project to foster a comfortable and inspiring learning environment. We drew inspiration from prestigious U.S. universities like Stanford and Princeton. A few faculty members, like Yuyun Lee (李玉雲) of dermatology, volunteered to help out with this project.


The Entrance to the College of Medicine.

The Entrance to the College of Medicine.
The cornerstone of this initiative was the creation of the Dean’s Garden Dean’s (院長花園 [定思園], Dìngsī Yuán). Bathed in sunlight, the garden boasted verdant lawns adorned with vibrant flowers and swaying trees, including Indian coral trees, bottlebrush trees, and horseshoe vines. Subtle touches, like a flower basket suspended from a lamp and a cozy rocking chair nearby, imbued the space with a European charm. Imagine sitting here, gently rocking in the breeze as birdsong fills the air – it would be a tranquil escape reminiscent of a European garden. The garden even featured a wooden deck, perfect for outdoor classes or faculty and student relaxation.
The name “Dean’s Garden” held a profound meaning. Inspired by the ancient Chinese philosophy text, The Great Learning (大學, Dàxué), it embodied the ideals of “calm deliberation” and, by extension, “setting aspirations, thoughtful reflection, and striving for excellence” (立定志向,思慮精詳,以臻至善). We were deeply grateful to the Rotary Club of Tainan (台南扶輪社) for their generous contribution that made this project possible. Interestingly, the English pronunciation of “Dean’s” is similar to the Chinese characters of “定思” (Dìngsī), further solidifying the connection. With this in mind, I declared that every Dean had the responsibility to preserve the beauty and integrity of the Dean’s Garden.

Donors of Dean’s Garden, including Dr. Kuochuan Kuo (郭國銓院長, the 4th from the left in the right photo) of Kuo's General Hospital 郭綜合醫院and Dr. Liangcheng Han (韓良誠醫師, the 4th from the left in the left photo ) and members of Tainan Rotary Club (台南扶輪社), viewing the completion of the Dean’s Garden. Biyu Zhou (周碧玉) and Vice Dean Yichian Lee (李益謙) are also present (the 1st and 2nd from left in the left photo).

Our beautification efforts extended beyond the garden. We refurbished various buildings, including the Dean’s office on the fourth floor, adorning them with captivating artwork. The artwork included the stunning “Apricot Grove” (“杏林”) mosaic mural and the “Medicine Wall” created by Professor Weijie Wang (王維潔) of the Department of Architecture. Additionally, we incorporated famous oil painting replicas from the Chi Mei Art Museum (奇美美術館), adding a touch of artistic prestige.

Left photo: The famous oil painting replica in the Dean’s reception room is donated by the Chi Mei Art Museum (奇美美術館).

Right photo: Vice Dean Yichian Lee (李益謙) uses the wooden deck in the Dean’s Garden (定思園) to give a lecture to the students.


Left photo: Dean Sung thanks Professor Weijie Wang (王維潔) (first from left in the left photo) for his painting "Apricot Lyceum" (杏子學園mosaic mural of Apricot Lyceum).

Right photo: The narrator is Vice Dean Yiqian Li (李益謙) (April 2005).

Left photo: the renovated Medical College Office Floor.

Right photo: returning alumni viewing the Graduate Alumni Showcase (校友櫥窗).
Enhancing Medical Education Through Arts and Humanities
Normally, we have art showcases, and occasionally there are oil paintings (such as European tour) and photography exhibitions (e.g., black-faced spoonbill). To cultivate a solid humanities foundation among our students, we implemented a series of arts and audiovisual programs. Led by Professors Mingliang Lai (賴明亮), Beichang Yang (楊倍昌), and Mr. Miao Su Zhong (鍾淼書) of Audiovisual Services (視聽中心), these initiatives aimed to create a stimulating and inspiring learning environment.
First, a “Music Appreciation Reading Group(音樂欣賞讀書會)” was established to provide a space for faculty and students to explore classical music. The success of this program led to the inclusion of opera appreciation (歌劇欣賞), Jazz Music Guide Appreciation (爵士樂導聆欣賞), and music tutorials (音樂導聆課程) led by Professor Zeiwei Liu (劉岠渭老師). Due to the overwhelmingly positive response from most faculty and students, the curriculum committee introduced an elective course called “Music and Medicine” a few years later, formally incorporating the exploration of music into the general curriculum (醫學通識課).
Additionally, a choir composed of medical students with a passion for music already existed within the medical school. Led by dedicated faculty members Zhisheng Lin (林志勝) and Chingchuan Liu (劉清泉), they improved their skills through regular practice and delivered inspiring performances throughout the campus. Their dedication extended beyond the campus as they volunteered in the community. As their sponsor, I witnessed firsthand their rehearsal process. Their performances at the annual year-end banquet were always a highlight. Seeing the joy on their faces after each performance was genuinely heartwarming.
Beyond music programs, Amin Huang (黃阿敏) introduced the practice of the Purifying Breathing Method (淨化呼吸法). The goal was to soothe the mind and body by focusing the mind, taking slow, deep breaths, and engaging in natural body movements to connect directly with nature and fresh air daily. This breathing technique attracted many faculty and staff, including myself.
More personally meaningful to me was the presence of a group of Christians (faculty, staff, and students) who formed a “Christ and Gospel Fellowship (基音團契) ” on campus. Their gatherings and community activities touched me, and I occasionally attended their meetings. Their love extended beyond the campus as weekday fellowship members volunteered at the hospital, offering comfort to patients and their families. This expression of faith proved to have a profound and lasting impact – patients and their families later founded the “Agape Vineyard Church (葡萄園教會)” in suburban Tainan. Witnessing them discover new strength and hope through faith during my visit was truly inspiring. It also brought back fond memories of my baptism.



Fellowship gathering and activities of "Christ and Gospel Fellowship (基音團契) ".

Throughout my tenure, Miao-shu Chung (鍾淼書) of the Audiovisual Center diligently documented the medical school’s activities, creating a valuable historical archive. These informative and educational videos were showcased on YouTube (listed at the end of this article).

Life Education and Role Models
Exposure to diverse perspectives and experiences in real-life situations is crucial for students’ growth. These measures aim to cultivate their empathy, communication skills, and global citizenship.
Life education is not confined by time or stage; its essence stems from individual experiences and role models in real life. We invited physicians like Professor Dafu Huang (黃達夫院長), Dr. Liangcheng Han (韓良誠醫師) to deliver speeches at graduation ceremonies to inspire the spirit of dedicated patient care and social service. In 2002, we further organized a “White Coat/Stethoscope Ceremony (白袍聽診儀式)” for fifth-year medical students before they embarked on clinical rotations. For the inaugural ceremony, we invited Emeritus Professor Juei-Low Sung (宋瑞樓), a revered medical educator and gastrointestinal disease specialist, to deliver a keynote speech, guiding medical students on how to become compassionate and skilled physicians.
Every summer, the medical school traditionally organizes medical students to provide medical consultations and services to remote communities with limited healthcare access, such as Chigu, Tainan County (台南縣七股) and Liouquei, Kaohsiung (高雄六龜). These experiences foster empathy and a sense of social responsibility. I visited them every year and listen to the students’ firsthand accounts. Their stories greatly touched me. Over time, we expanded the scope of volunteer work to include Malawi (馬拉威), a landlocked country in southeastern Africa. Upon returning from Malawi, students shared their experiences. They created moving slideshows and videos with music, depicting the harsh reality of limited resources and healthcare services and expressing their feelings. They expressed disbelief at the living conditions faced by the people of Malawi. Their deep empathy reaffirmed my belief that these students possess the compassion and dedication needed to become doctors or nurses.

Left photo: Left: Dean Sung encouraged students to provide medical services in remote areas.

Right photo: Dean Sung, second from right, served as a guest lecturer, is welcomed to the University of Mississippi Medical Center by, from left, John Payne, Associate Professor, Dr. Ing K. Ho, Dean of Graduate Study, and Dr. Richard de Shazo, Chairman, Department of Internal Medicine.

Professor Dafu Huang (黃達夫院長), the invited speaker of the June 2006 graduation ceremony (second from the right) with (雀櫻) on the left and Juanjuan (娟娟) on the right.

We invited Academician Rui Lou Song (宋瑞樓院士) to address to the 5th-year medical students before they started clinical clerkship, the White-Coat/Stethoscope Ceremony, 2002.

We invited Academician Rui Lou Song (宋瑞樓院士) to address to the 5th-year medical students before they started clinical clerkship, the White-Coat/Stethoscope Ceremony, 2002.
As always, the “Huang Dafu Medical Education Promotion Foundation (黃達夫醫學教育促進基金會)” annually sponsors a selected fifth-year student for a three-month summer internship at Duke University. Past recipients have consistently praised Duke University’s patient-centered holistic healthcare approach and treasured the valuable experience gained. Upon graduation, some were drawn to pursue their medical careers in the United States. For instance, Chia-Wen Lee (李加文), a 2005 medical school graduate who was selected for a summer internship at Duke University, obtained a Master’s in Public Health from the University of Michigan and completed a pediatric residency program at Michigan Children’s Hospital. A few years later, in 2018, upon my recommendation, she joined the pediatric practice at Stanford Health Care in San Jose, California, where she continues to work today.

Chia-Wen Lee and husband, Frederic at Filoli Estates and Garden, Woodside, CA, 2023.

Left photo: Dean Sung teaching ECG at the bedside.

Right photo: Dean Sung presenting a souvenir to Professor of Clinical Internal Medicine, Department of General Internal Medicine, Ohio State University School of Medicine, Harrison G. Weed(Nov. 25, 2005).
Cultivating English Language Skills
Recognizing the critical role of English in today’s globalized medical field, we have implemented several initiatives. We collaborated with the popular Studio Classroom (空中英語教室), founded by dedicated educator Doris Marie Brougham (彭蒙惠). Their programs are broadcast daily at noon. Additionally, we hired Mr. Mark to provide “Live English Conversation” classes every Tuesday evening, either in classrooms or at various venues such as Starbucks, creating a dynamic learning environment. Furthermore, we encouraged faculty, especially those who recently completed research training in the United States and other English-speaking countries, to deliver lectures in English.
To enhance the practical application, we annually sponsored (airfares provided) 30 medical students for summer clinical observations at several medical centers in the United States, including Tulane University, the University of Mississippi, the University of Illinois, and Baylor College of Medicine at the Texas Medical Center in Houston.

Mark and Watson English Club.

Mark and Watson English Club.
Embracing Diversity and Expanding Horizons
With a vision of international cooperation, Vice Dean Lien-I Hor (何漣漪) pioneered an initiative in 2005 to admit international students into our medical school. Paul Bosawai Popora, from the Solomon Islands, became our first such student. Subsequently, we enrolled several more medical students from the South Pacific Islands.
Despite the initial challenges of language and culture, Paul received unwavering support from his classmates and teachers throughout his nine-year journey, graduating in 2014. Paul now runs the Sape Clinic in his native Solomon Islands, a clinic staffed with three doctors providing primary medical services, including internal medicine, pediatrics, and surgery. Notably, the Sape Clinic has become a cornerstone of healthcare in his homeland. Paul has also become a respected community leader. Recently, he was even nominated by his parliament and won the election to become a member of parliament. Paul’s example embodies the success of our program.

Paul Bosawai Popora before graduation at NCKU Medical College (the third from the left) and his current Sape Private Medical Clinic in Solomon Islands.

Paul Bosawai Popora before graduation at NCKU Medical College (the third from the left) and his current Sape Private Medical Clinic in Solomon Islands.
To further support international students, researchers, and visiting doctors in our affiliated hospitals, we established a dedicated international office and recruited Jui Chen (陳瑞楨) as our liaison officer. Academically, we actively promoted cross-cultural exchanges through faculty and research collaborations with renowned institutions such as the University of Hong Kong and Lithuania’s Vilnius University (Vilniaus Universitetas), the oldest and largest institution of higher education in Lithuania. To help our students gain valuable overseas clinical experience, I led teams on visits to several medical centers abroad and personally visited the deans of medical schools at Tulane University, the University of Mississippi, and Baylor College of Medicine, paving the way for student internships.

Light photo: signing an academic cooperation agreement with the University of Hong Kong (Jan. 10, 2002).

Right photo: Academician Yinggang Ho (何英剛院士) and Vice President of University of Mississippi Medical Center Dr. Wallace Conerly visited the NCKU Medical Center to sign an education cooperation agreement (Apr. 23, 2002).

Left photo: The Department of Nursing and the Hong Kong Polytechnic University signed an exchange and cooperation plan in our hospital. Dean Sung and Chair of the Department of Nursing Meizhi Huang (黃美智) presented souvenirs to representatives of the Hong Kong Polytechnic University (Nov. 09, 2006).

Right photo: Research and development cooperation agreement signed with Lithuania’s Vilniaus Universitetas, 2006.
Unforgettable Encounters, Inspiring the Future
During my tenure as Dean, I had the privilege of meeting and hosting many distinguished international physicians and scholars. I will never forget meeting the renowned cardiovascular surgeon and medical educator Dr. Michael DeBakey (1908-2008) at Baylor College of Medicine in 2004. He was 95 years old at the time. DeBakey invented the roller pump while at Tulane Medical School in the 1930s. It later became a core component of the heart-lung machine, which took over the functions of the heart and lungs during surgery by supplying oxygenated blood to the brain – helping to usher in the era of open-heart surgery. Additionally, he was a pioneer in the implantation of artificial hearts and assist devices. As an immigrant from Lebanon, I asked him what mattered most to him about his life and career in America. Without hesitation, he replied, ” Freedom and democracy”.

Dean Sung, Chair Meizhi Huang (黃美智)and Chongjie Luo (羅崇傑) visited the DeBakey Heart Center of Methodist Hospital and took a photo with Dr. Michael DeBakey (second from left), Dr. Arthur Ericcson, Dr. Stone Cao and Ruthy Khawaja (Nov. 3, 2003).
Dean Sung and facultymembers including Chihe Lin (林其和) visiting Harvard Medical School, 2006.
Dean Sung and facultymembers including Chihe Lin (林其和) visiting Harvard Medical School, 2006.
Among the esteemed scholars who visited our National Cheng Kung University Medical College were several Nobel laureates. These luminaries included John R. Vane (Nobel Prize in Physiology or Medicine, 1982), Ferid Murad (Nobel Prize in Physiology or Medicine, 1998), and Aaron Ciechanover (Nobel Prize in Chemistry, 2004).
John Vane’s groundbreaking discovery of aspirin’s mechanism of action, specifically its ability to block prostaglandin synthesis, revolutionized our understanding of its clinical pharmacology. Ferid Murad’s identification of nitric oxide (NO) as a signaling molecule in the cardiovascular system has had a profound impact on cardiovascular medicine.
Aaron Ciechanover’s groundbreaking work on the ubiquitin-proteasome system, which regulates protein degradation, has significantly advanced our understanding of cellular processes. This work provides a fascinating counterpoint to the research of Arthur Kornberg, who focused on protein synthesis.
During his visit to National Cheng Kung University in 2007, Aaron Ciechanover captivated us with his vitality, friendliness, and genuine interest in our institution. In addition to delivering insightful lectures, he engaged with students and even assisted the then-provost in fundraising efforts. Later, I had the privilege of working with him on the Scientific Advisory Board of Hwa Chung Science and Technology University in Wuhan, China, which deepened my appreciation for his character and intellect. The grand ceremony honoring him with an honorary doctorate from National Cheng Kung University was a memorable experience. It was an honor to introduce such a distinguished scholar to our university community.
Like Arthur Kornberg of Stanford University, Aaron Ciechanover serves as an inspiring role model for our faculty and students.

Left photo: Prof. John Vane(1982 Nobel Laurette)(Oct. 24, 2001).

Right photo: Prof. Ferid Murad(1998 Nobel Laurette)(Jan. 22, 2002)visiting Dean Sung.

Dean Sung with distinguished guests participating in a series of academic activities of the Global Innovation Center of Excellence hosted by our school, including 2004 Nobel Prize winner Aaron Ciechanover, Academician Jingwu Zhu朱經武院士, Academician Mingzhao Lai (賴明詔院士) and Dr. Daxuan Feng (馮達旋博士) (Dec. 12, 2006).

Dean Sung with distinguished guests participating in a series of academic activities of the Global Innovation Center of Excellence hosted by our school, including 2004 Nobel Prize winner Aaron Ciechanover, Academician Jingwu Zhu朱經武院士, Academician Mingzhao Lai (賴明詔院士) and Dr. Daxuan Feng (馮達旋博士) (Dec. 12, 2006).
Cultivating Alumni Connections
As a vice president of the university, I have traveled to Hong Kong, Macau, and Malaysia to connect with our overseas alums. I was delighted to see that most of our alums have successfully started their businesses and have made significant contributions to their respective societies or countries. In 2005, I met alumnus Tony Huang, who graciously took me to visit the renowned Happy Valley Racecourse in Hong Kong, one of the world’s most famous racecourses, where he served as Director. At the reception dinner, I gave a speech outlining the future direction of our alma mater and expressing warm greetings to the alumni.
I have visited Malaysia twice – Kuala Lumpur (吉隆坡), Penang (檳城), and nearby areas. I met many alums, including a high school classmate. Despite Malaysia’s severe anti-Chinese policies, they were all quite successful businessmen in the fields of electrical, mechanical engineering, and construction. I was surprised to see that residents of Penang spoke the same Minnan dialect as people in Taiwan. I also visited Chiming High School (啟明高中), which has a cooperative education program with our National Cheng Kung University. Before I left, the alumni asked me to give a special lecture on health issues, particularly those related to the cardiovascular system. To this end, I metaphorically used a punning title “How to Be a Good Heart Person [如何成為一個好心人].” Seeing so many listeners in the gymnasium, all of whom were our overseas compatriots, filled me with strong emotion.

When I arrived at the medical school, the medical school alumni association was virtually nonexistent. With the help of Yue-Jen Ho (何月仁老師) and alums Dr. Joseph Yu (余約瑟) and Chien-Ming Lee (李健明), I took on the task of reorganizing the association, with Dr. Joseph Yu volunteering to serve as the president, and myself providing office space. Afterward, we encouraged medical school graduates to maintain strong connections and support each other in their continued service to society. We established branch organizations in the north, central, and south of Taiwan. I was pleased to be able to give speeches at several of these gatherings. Based on my experience at Stanford University, alumni are the backbone of promoting the development of medical schools and advancing medical education.




In October 2003, our alumni returned to the Alma mater for the first time.


Five alumni including Dr. Wei Chen (陳偉) accompanied Kuei and I to climb Jade Mountain (2005).

NCKUMC alumni from the north gathering at the International Conference Center of the National Taiwan University School of Medicine ( June 2007).
Transformation of National Cheng Kung University Medical College: A Legacy of Growth and Innovation
As Dean, I noticed that the academic promotion system was unreasonable and unfair. Each candidate had to be voted on by a promotion committee, which consisted of approximately 15 elected members from different disciplines. Therefore, promotions were judged by a majority of members whose specialties differed from those of the candidates. Such a system was easily susceptible to personal relationships and political manipulation. Additionally, the evaluation system emphasized academic impact factors (high-impact publications). Compared to basic sciences, departments such as nursing, rehabilitation, and public health were at a considerable disadvantage. In the United States, for academic promotion, candidates are evaluated by an ad hoc committee consisting of 4 to 5 faculty members with related or similar specialties. Since I could not change the structure of the promotion committee, I consulted with several vice deans. Later, we implemented a new evaluation system that divided the evaluation into three different categories based on the applicant’s primary interest or focus: research, teaching, or service.
To enrich our faculty, the medical school established new institutes during my tenure, including two doctoral programs of the Institute of Industrial Hygiene and Environmental Medicine (工業衛生學科暨環境醫學研究所) and the Institute of Health Care Science健康照護科學研究所, and five master’s programs of the Institute of Stomatology (口腔醫學研究所), the Department of Physical Therapy (物理治療學系) the Department of Occupational Therapy(職能治療學系), the Institute of Gerontology (老年學研究所), and the Institute of Clinical Medicine for Physicians(臨床醫學研究所碩士在職專班).
Notably, due to the aging population in Taiwan, Dr.Liang-Cheng Han (韓良誠醫生) and I visited Minister of Education Cheng-Tseng Tu杜正勝教育部部長. Minister Tu personally approved our request to establish the nation’s first Institute of Gerontology and generously added three faculty positions to the medical school (a total of 6, with the other three provided by the university).
Furthermore, we actively recruited scholars from both domestic and international institutions, such as Rubin Lu (陸汝斌) (psychiatry), Sheng-Nan Wu (吳勝男) (cellular electrophysiology), Charity Tsai (蔡淳娟) (pediatrics), Christina Lin Chang (張玲) (cancer research), and Chingho Hsieh (謝清河) (stem cell). We also promoted the professional development of existing faculty members. A few examples include:
- Da-Bin Xie (謝達斌) (dentistry), with the help of Professor Chen-Sheng Yeh (葉晨聖) of the Department of Chemistry in the College of Science (a research assistant hired by the medical school working in the College of Science), successfully developed nanomedicine, which has gained both domestic and international recognition.
- Ming-Hsiang Chang (張明𤋮), a Wang Ming-Ning Memorial Foundation Award (王民寧醫療科技貢獻獎) recipient, is dedicated to researching and developing new drugs for the treatment of osteoporosis, rheumatoid arthritis, and pancreatic cancer, and has received the highest amount of sponsorship for Cheng Kung University from an international pharmaceutical company.
Keshi Chao (趙可式) is a leading advocate for hospice care in Taiwan. She received the 14th Medical Dedication Award for Special Contributions (醫療奉獻獎「特殊貢獻獎」2004) and has authored books such as Physicians and Life and Death (醫師與生死) and Peaceful Accompaniment (安寧伴行). Her significant contributions to the field have earned her the title of “Mother of Hospice Care” (安寧療護之母) in Taiwan.

Keshi Zhao (趙可式) received the 14th Medical Dedication-"Special Contribution Award" (2004).
- Junchiang Yu (余俊強) led the Laboratory Animal Center (實驗動物中心) of our College and strived to be appointed as the Director of the National Laboratory Animal Center (國家實驗動物中心) of the National Applied Research Laboratories (國家實驗研究院).
- Bozhang Lee (李伯樟), Chair of our College’s Department of Surgery, became the superintendent of Tainan Provincial Hospital and was then appointed head of the National Health Insurance Administration (衛生福利部中央健康保險署), which he served for six years and 8 months.
- Meixia Chen (陳美霞), Chair of the Institute of Public Health, served as Editor of NCKUMC News Letters (成大醫訊) and continued her work on public health issues through Taiwan Public Health Promotion Association (臺灣公共衛生促進協會) after retirement.
- Mingzhe Tang (湯銘哲), from Executive Vice Dean of the College to Provost of the University (大學教務長), President of Tunghai University (東海大學), to his never-ending research in regenerative medicine, has gained widespread social recognition for his strong support of patients with hereditary epidermolysis bullosa (泡泡龍症).
- Junzhong Wu (吳俊忠), from a research institute director and Vice-dean of the College to the current Dean of the College of Medicine and Health at Asia University (亞洲大學醫學與健康學院院長), is dedicated to research and teaching and also devotes time and energy to promoting environmental protection movements.
Fostering a Culture of Excellence in Teaching
We set up a Teacher Development Center. Workshops are held every year in beautiful places such as West Lake Resortopia (西湖渡假村), Miaoli to promote teacher development. These retreats provided space for relaxation, reflection, curriculum revision, and teaching skill enhancement. Renowned educators like Mingde Lai (賴明德), Junzhong Wu (吳俊忠), and Keshi Zhao (趙可式) shared their unique teaching methods. Additionally, I actively solicited feedback on the College’s future direction during these retreats.
We encouraged our faculty returning with doctoral degrees from the U.S. and U.K. to deliver lectures in English. We even organized a faculty visit to our sister institution, the University of Hong Kong. This institution employs English for medical lectures but retains Cantonese for daily communication, mirroring Taiwan’s situation. Each year, a committee of faculty and students selected outstanding educators for teaching awards.

Left photo: Prof. John Vane(1982 Nobel Laurette)(Oct. 24, 2001).


Faculty Retreat at Tachikawa Fishing Ground (立川漁場).


Faculty Retreat at West Lake Resort (西湖渡假村) April 15-16, 2005.


Professor Lien-I Hor (何漣漪) (left photo) and Professor Keshi Zhao (趙可式) (right photo) receiving the Outstanding Teaching Award.

From Vision to Reality: The Birth of the College of Bioscience and Biotechnology and Cooperation with Academia Sinica
The members of the Biotechnology Center (生物科技中心) primarily consisted of faculty from the Colleges of Medicine, Science, Engineering and Electrical Engineering. We met once a month to discuss ongoing projects and future directions. As the center director, drawing on my experience at National Chiao Tung University (國立交通大學), I proposed an ambitious suggestion – to transform the center into a new college of the university (生物科技學院). The members responded very positively. Subsequently, we worked together diligently to write a proposal and submitted it to the university and the Ministry of Education. Executive Vice Dean Mingzhe Tang (湯銘哲) and Vice Dean for Research Lei Huanyao (黎煥耀) devoted a great deal of time and effort throughout the process. After several months, the Ministry of Education finally approved a grant of NT$80 million to assist us in establishing the College of Bioscience and Biotechnology (生物科技學院), opening up another new path for faculty career development within the university.
To promote research cooperation and academic exchange between National Cheng Kung University and Academia Sinica (中央研究院), in the field of agriculture, I made numerous trips to the Tainan County Government and Academia Sinica to negotiate. Su Huan-Chih (蘇煥智), then the magistrate of Tainan County (台南縣長), generously offered to provide a free plot of land in the science park for National Cheng Kung University and Academia Sinica to build a bridge type twin towers (橋式雙塔). Yuan-Tseh Lee (李遠哲), then president of Academia Sinica (中央研究院院長), personally attended the groundbreaking ceremony. This cooperative project enhanced the interaction between NCKU faculty, students, and researchers both domestically and internationally and raised the reputation of NCKU.
A Student’s Spark: Embracing Mathematical Cardiology
Once upon a time, a student posed a thought-provoking question about the role of mathematics in medicine, sparking a new research avenue for me. This inquiry led me to reflect on the Luo-Rudy dynamic model, a sophisticated mathematical framework that simulates the cellular electrophysiology of heart cells, specifically guinea pig ventricular myocytes. It reminded me of Professor Ching-Hsing Luo (羅錦興) in our engineering school. In 1994, while pursuing his Ph.D. at Case Western University, Luo and his mentor, Professor Rudy Yoram, developed the original Luo-Rudy dynamic model (a dynamic model of the cardiac ventricular action potential) (Ref. 70). This model provided a detailed description of the electrophysiological activity of a single mammalian ventricular cell, focusing on membrane sodium and potassium currents and the regulation of intracellular calcium concentration.
With Professor Luo’s expertise, I invited Sheng-Nan Wu (吳勝男), a cellular electrophysiologist at NCKUMC, to form a research group embarking on a collaborative journey. Driven by my desire to learn more, we visited Professor Luo’s doctoral advisor, Professor Yoram Rudy, who had moved to Washington University in St. Louis. . Professor Rudy’s generosity and hospitality were impressive. He spent two days introducing us to his research team and their current projects and gave us valuable advice.
Upon returning, we quickly reproduced and updated the Luo-Rudy dynamic model of ventricular myocytes, with a particular focus on adding detailed simulations of intracellular calcium homeostasis and β-adrenergic-receptor stimulation. Our initial plan focused on the mechanisms of hypokalemia-related ventricular arrhythmias in Andersen-Tawil syndrome. This study was published in the American Journal of Physiology in 2006 (Reference 71). It greatly deepened my understanding of how ion currents shape the complex morphology of the electrocardiogram and how ion channelopathy can lead to arrhythmias.
The above study also helped me understand the mechanisms underlying the generation of early and delayed after-depolarizations and their roles in triggering arrhythmias. This knowledge solidified my confidence in the classification system of ventricular tachycardias that I proposed, which is based on the differences in the initiation, termination, and response to drugs such as verapamil and propranolol of ventricular tachycardia (Refs 37, 38).
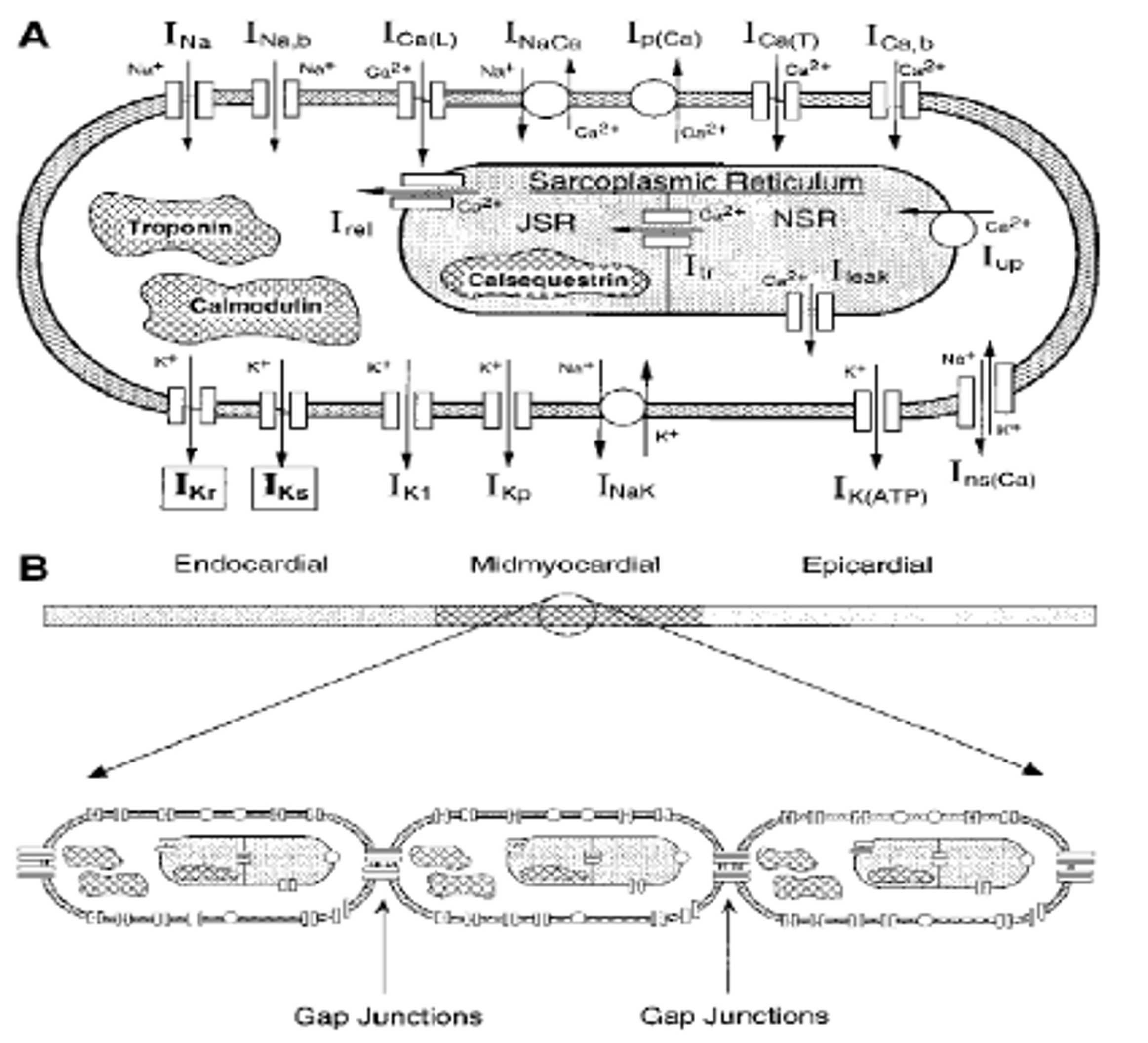
1-Dimensional Multicellular Strand (Luo-Rudy Model) (From PC Viswanathan, RM Shaw, Y Rudy. Effects of Ikr and Iks Heterogeneity on Action Potential Duration and its Rate-Dependence: A Simulation Study. Circulation. 1999;99:2466-2474 with permission).

Dean Sung, Professor Jinxing Luo (羅錦興, first right), and Professor Shengnan Wu (吳勝男, first left) with the host Professor Yoram Rudy (second right) at Washington University in St Louis (WUSL), Bioelectricity and Arrhythmia Center (Sep. 6, 2006).
Reflecting on My Tenure
Despite my dedication to my roles as Dean of the College and Vice President of the University, I still feel regret or a sense of missed opportunities when looking back on certain aspects of my tenure.
The passing of Research Vice Dean Huanyao Lei (黎煥耀) from pancreatic cancer was a tremendous loss to the college, the university, and the broader medical community. His leadership and expertise were extremely valued.
Together with the chair of the Department of Nursing, Meizhi Huang (黃美智), I envisioned a strategic collaboration with the neighboring Tainan School of Professional Nursing (台南護理專科學校) to elevate our Department of Nursing into a College of Nursing at National Cheng Kung University. This plan reflected a nationwide trend, as seen in institutions such as National Taiwan University and Kaohsiung Medical University. However, the plan was delayed due to concerns from the faculty of the nursing college. Specifically, they worried that the unfair academic promotion system established by the university and the Ministry of Education would negatively impact their future career development.
According to the regulations of the Ministry of Education, Chiang Gao’s (高強) second consecutive term required a faculty vote. When seeking his support for the appointment of the affiliated hospital superintendent, he was hesitant to support my choice due to concerns about opposition from certain factions of the faculty, especially those in the hospital. As a result, I offered to resign twice as vice president. However, President Kao was indecisive about accepting my resignation, possibly fearing potential negative publicity. In retrospect, a more open dialogue may have led to a more satisfactory solution.
Finally, I admit that agreeing to appoint Chih-Hong Chen (陳志鴻) as the superintendent of the affiliated hospital was not the best decision. Professor Bing-Wen Lin (林炳文) was an outstanding surgeon with extraordinary sincerity and humility and would have been a better choice. Even during his illness, his warm greetings when I returned to the college still lingered in my memory. His passing was another significant loss to the college, the university, the medical community, and society.
A Legacy of Lasting Impact
Before leaving National Cheng Kung University (NCKU), Vice Dean Mingzhe Tang (湯銘哲) and I raised NT$15 million. This substantial sum, donated by an anonymous artist and Chonghua Cheng (鄭崇華) of Tai-Da Electronic Enterprise [台達電子企業], was specifically designated for the construction of a clinical skills training center (臨床技能培訓中心) within the medical school. Our shared vision and dedication also successfully secured additional funding from the Ministry of Education, which played a significant role in the expansion of the affiliated university hospital. These initiatives undoubtedly strengthened the capabilities of the NCKU Medical Center (成大醫學中心) with their tangible and substantial impact, paving the way for future growth and innovation.
We also changed the regulations for the renewal of the dean of the medical school. Initially, this process required a vote from all faculty members and was susceptible to factional manipulation. Specifically, the approval of a second term should be evaluated by a nine-member committee, including three faculty representatives (basic sciences, clinical medicine, and the in-between), one student representative, the dean of the College of Science, the dean of the College of Engineering, and external representatives (such as the dean of the National Taiwan University College of Medicine and the president of Kaohsiung Medical University), who would assess the current dean’s performance over the past three years. This change was the result of our collective understanding of the need for a more streamlined and efficient process, reflecting our commitment to continuous improvement.
References
(70) Luo CH and Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circulation Research, 1994;74:1071–1096.
(71) Sung RJ, Wu SN, Wu JS, Chang HD, Luo CH. Electrophysiological mechanisms of ventricular arrhythmias in relation to Andersen-Tawil syndrome under conditions of reduced IK1: A simulation study. American Journal of Physiology-Heart and Circulatory Physiology, 2006;291:H2597-H2605.
Miao Su Zhong (鍾淼書)’s Video Documentaries:
https://youtu.be/j9Utms-6xVA?si=u6KWAyADKXW4g0Fg
https://www.youtube.com/watch?v=GJX9XXr2W2Y&list=PLv_0H46yed-7QOCNaKN7xGiv1IFIKt9px&index=23
https://www.youtube.com/watch?v=4z15bXcbCeI&list=PLv_0H46yed-6FFh4xKbwAENo8kzB-p3qI&index=9
https://www.youtube.com/watch?v=K86LHSkpcXo&list=PLv_0H46yed-6FFh4xKbwAENo8kzB-p3qI&index=11
https://www.youtube.com/watch?v=n4Aox04zCUg&list=PLv_0H46yed-5bSku3LiV4TtnOvA-Y7Ips&index=1
https://www.youtube.com/watch?v=X1uT60qHLrw&list=PLv_0H46yed-5bSku3LiV4TtnOvA-Y7Ips&index=9
https://www.youtube.com/watch?v=t_05W-ELiRQ&list=PLv_0H46yed-5bSku3LiV4TtnOvA-Y7Ips&index=21
https://www.youtube.com/watch?v=X1uT60qHLrw&list=PLv_0H46yed-5bSku3LiV4TtnOvA-Y7Ips&index=9
https://www.youtube.com/watch?v=t_05W-ELiRQ&list=PLv_0H46yed-5bSku3LiV4TtnOvA-Y7Ips&index=21
https://www.youtube.com/watch?v=ATXIoqI1TIA&list=PLv_0H46yed-5FIUG2F3c_iCmm4M2qUTQJ&index=7
https://www.youtube.com/watch?v=5NrbM4aqVkU&list=PLv_0H46yed-5FIUG2F3c_iCmm4M2qUTQJ&index=9
https://www.youtube.com/watch?v=UiRKOKzRH1I&list=PLv_0H46yed-5FIUG2F3c_iCmm4M2qUTQJ&index=10
https://www.youtube.com/watch?v=7FnjxWQroqo&list=PLv_0H46yed-7V7lojn4JzLJBa5n_hodlr&index=1&t=1s
https://www.youtube.com/watch?v=EoBMWUobgk8
https://www.youtube.com/watch?v=o3Bnl3JyAjY
National Central University (September 1, 2007 – June 30, 2012)
In September 2007, President Luo-Chuan Lee (李羅權) of National Central University recognized my expertise and offered me a position as a distinguished professor in the Institute of Life Sciences, allowing me to continue pursuing my passion for cardiac electrophysiology research. He also generously offered me the position of vice president of the university. However, I was aware of the political complexities of the school and politely declined his offer, choosing to prioritize research and teaching.
Building a New Research Team and Fostering Collaboration
At the Institute of Life Sciences, I organized a weekly guest speaker program, taught a course on electrophysiology, and supervised graduate students. Additionally, every Wednesday morning, I conducted bedside teaching with interns and resident physicians at Li-Shing Hospital at Taoyuan (桃園壢新醫院), which is not too far from the campus.
Recognizing the increasing competitiveness of computational cardiac electrophysiology, I collaborated with two mathematics professors, Chu-Pin Lo (羅主斌) and Hui-Chun Tien (田惠君) from Providence University (靜宜大學). Despite the geographical distance, we met once a week and held meetings with students from both universities to discuss and exchange ideas.
Unveiling the Mechanisms of Timothy Syndrome
Our initial research focus was on Timothy syndrome (TS), a rare autosomal dominant disorder affecting young individuals, characterized by dysfunction of multiple organ systems (such as long QT syndrome type 8, structural heart defects, syndactyly, autism, and immune system defects). TS has mutations in the CACNA1C gene, leading to a near-complete loss of function of the voltage-dependent inactivation (VDI) of the L-type calcium current (ICa, L; Cav1.2), resulting in a “gain of function” of the L-type calcium channel and intracellular calcium overload.
We combined the existing updated Luo-Rudy model with Markov models based on the structure of the ICa, L (Cav1.2) channel, and ryanodine receptor 2 (RYR2), enhancing the details of ion channels and intracellular calcium homeostasis. The improved model enabled us to simulate various clinical scenarios in the hearts of TS patients. Our simulations showed how different degrees of the G406R Ca(v)1.2 channel mutation disrupt intracellular calcium handling, leading to a progressive prolongation of action potential duration (APD) and QT interval, followed by early and delayed afterdepolarizations and an increase in transmural dispersion of repolarization, which may cause arrhythmias due to enhanced triggered activity and reentrant substrates. We also observed that increased adrenergic stimulation (β-adrenergic stimulation) exacerbated these effects. Furthermore, our study suggests that not only the calcium channel but also the sarcoplasmic reticulum Ca2+ATPase uptake current was a promising therapeutic target.
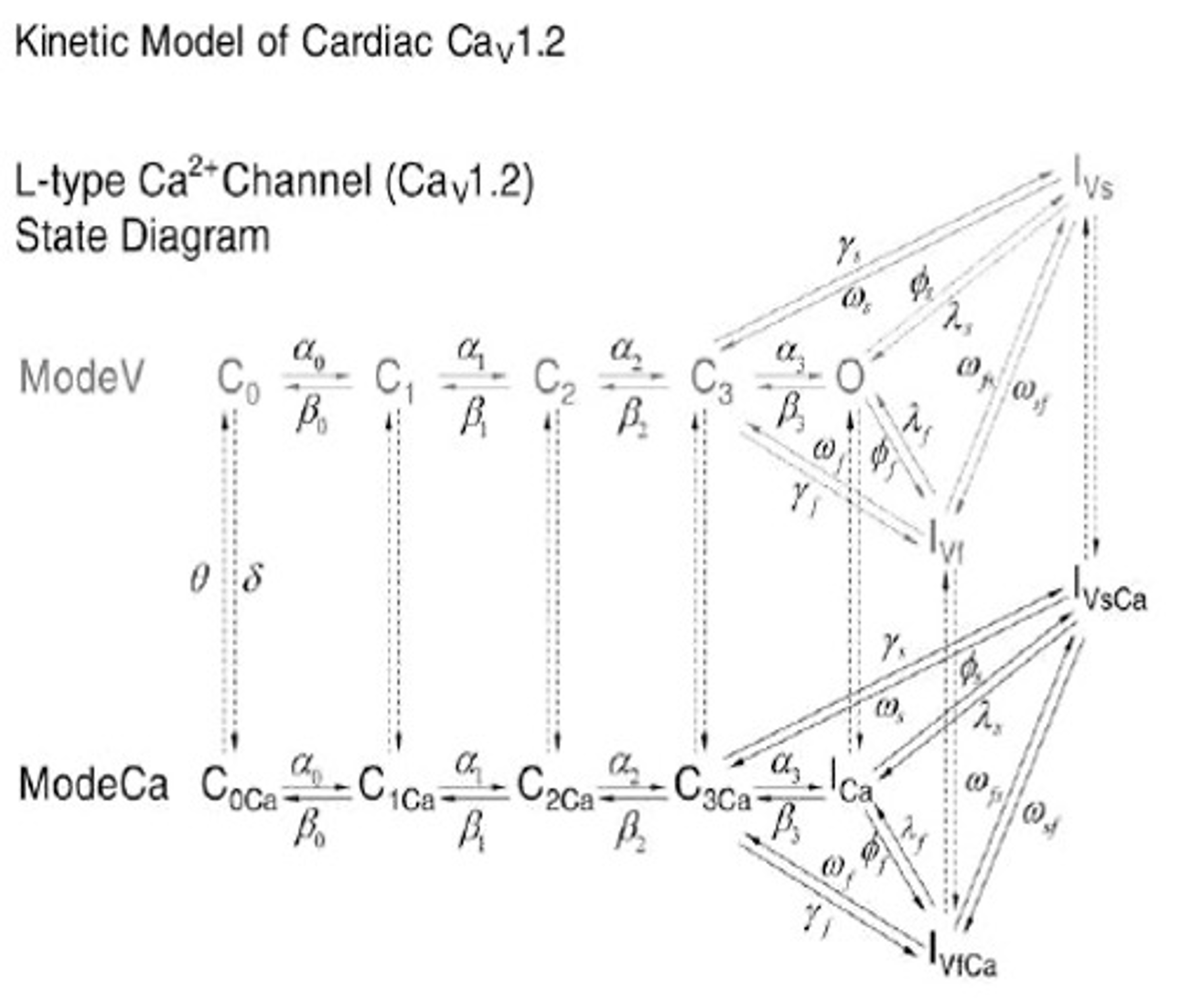
State diagram of the L-type Ca2+ channel Markov model. The upper tier (ModeV) represents a voltage-dependent inactivation gating mode (VDI) and the lower tier (ModeCa, black) represents a Ca2+-dependent inactivation gating mode (CDI). (From G.M. Faber, J. Silva, L. Livshitz, Y. Rudy. Kinetic Properties of the Cardiac L-type Ca2+ Channel and its Role in Myocyte Electrophysiology: A Theoretical Investigation. Biophys J. 2007 Mar 1;92(5):1522-43 with permission).
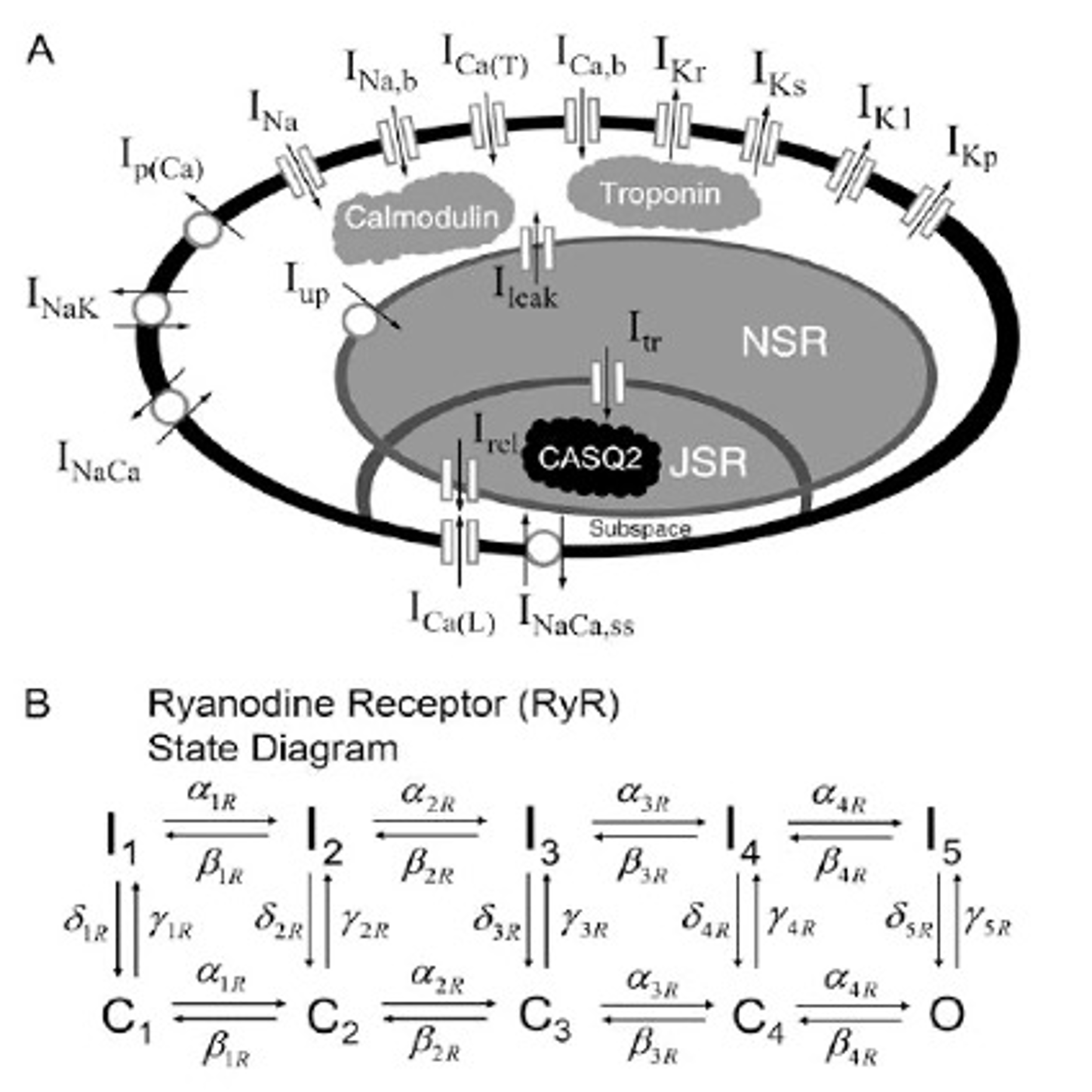
A. Schematic for the four-compartment model (bulk myocardium, JSR, NSR, and subspace) of the guinea pig ventricular myocyte. B. State diagram of the ryanodine receptor, RyR. (From G.M. Faber, J. Silva, L. Livshitz, Y. Rudy. Kinetic Properties of the Cardiac L-type Ca2+ Channel and its Role in Myocyte Electrophysiology: A Theoretical Investigation. Biophys J. 2007 Mar 1;92(5):1522-43 with permission).
Publication and Recognition
The American Heart Association accepted our research results for presentation as an abstract at its annual meeting. After receiving positive reviews with constructive criticism, we dedicated an additional 3-4 months to refine the manuscript and address the reviewers’ comments. Our research was ultimately published as a lead article in the October 2009 issue of the American Journal of Physiology (Ref. 39), accompanied by an editorial (Ref. 72).
The editorial praised our work for establishing a “sympathetic model of L-type Ca2+ channel-triggered arrhythmias,” laying the groundwork for a more comprehensive model integrating cellular signaling and cardiac electrophysiology. This breakthrough could facilitate a deeper understanding of inherited ion channelopathies and the impact of β-adrenergic receptor stimulation on complex cardiac conditions like heart failure. We believe that this research approach can be applied to more sophisticated simulation models to elucidate the mechanisms underlying arrhythmias and identify potential molecular targets for pharmacological or genetic interventions, further validating the significance of our work.
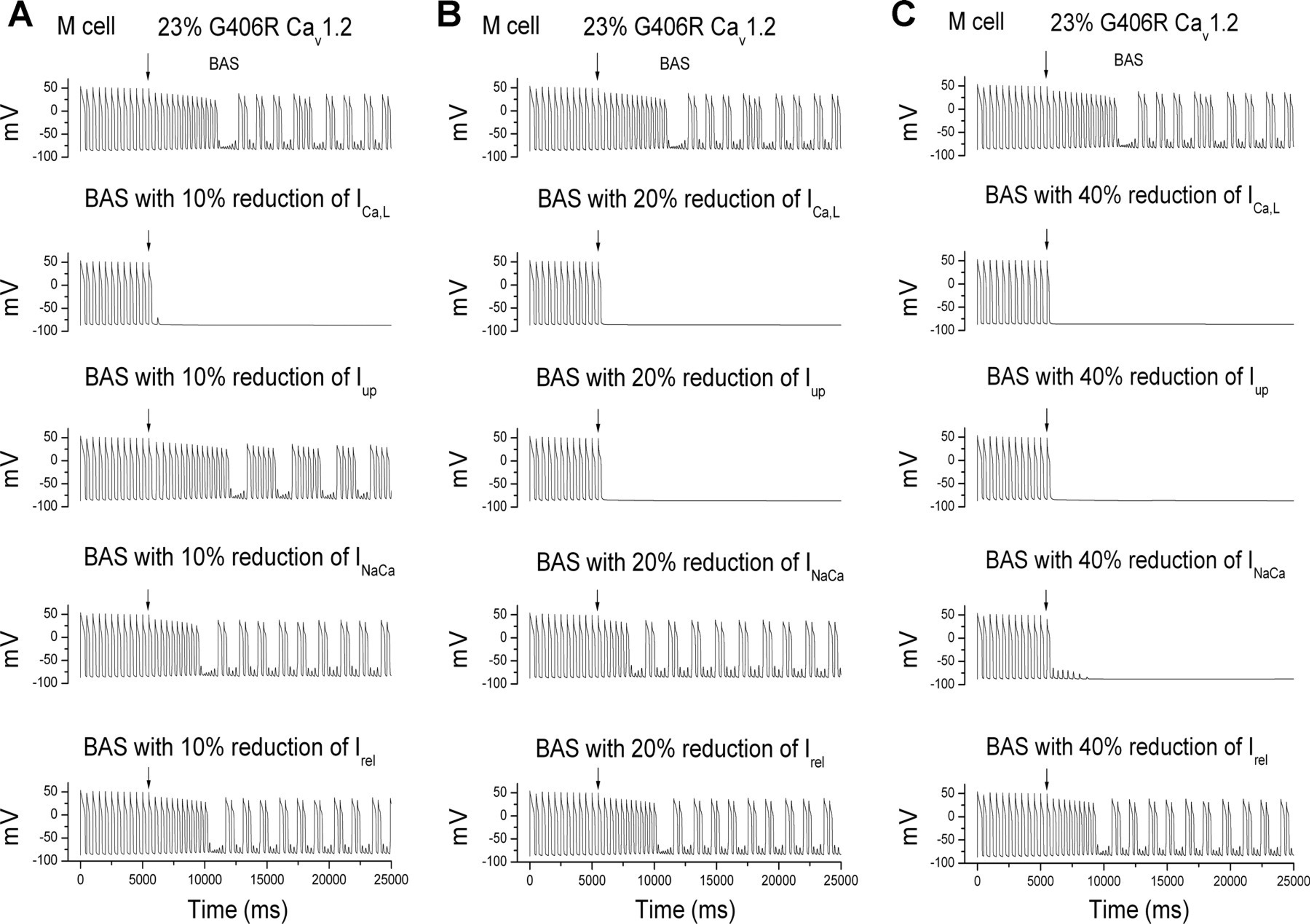
Development of pacing-induced DAD-mediated triggered activity in the ventricular myocyte containing 23% of G406R Cav1.2 channels under the influence of BAS (β-adrenergic receptor stimulation). A: at control, pacing for 12 stimuli (last stimulus denoted by a solid arrow) at 2.0 Hz in the M cell containing 23% of G406R Cav1.2 channels induced no DADs. B: after the addition of BAS, pacing for 12 stimuli (last stimulus denoted by a solid arrow) at the same frequency provoked the onset of DAD-mediated triggered activity lasting for 13 beats (cycle length: 417 ms), during which there was gradual depolarization of the membrane potential (Vm increased from 87.4 to 81.1 mV), followed by transformation into sustained DAD activity with intermittent captured beats (1st beat denoted by a dotted arrow). Portions of the tracing underlined as (1) and (2) are amplified to show intracellular ionic events in Figure below, A and B, respectively (Reference 39, American Journal of Physiology with permission).
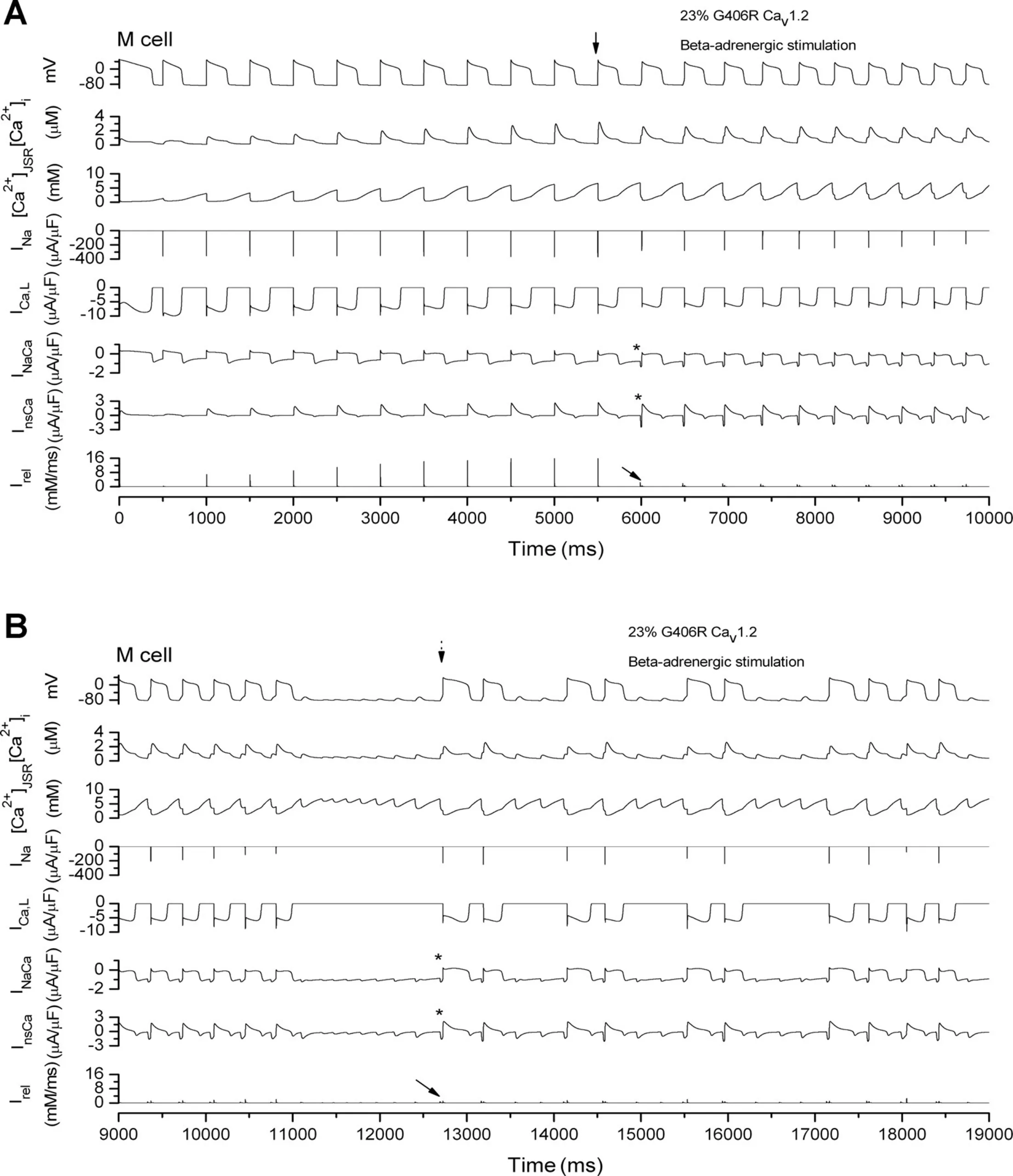
Intracellular ionic events underlying induction of pacing-induced DAD-mediated triggered activity with 23% of G406R Cav1.2 channels under the influence of BAS. A and B correspond to the underlines (1) and (2), respectively, as shown in Fig. above. Membrane potentials (mV) are shown at the top (see text). [Ca2]i, intracellular Ca2 concentration; [Ca2]JSR, junctional sarcoplasmic reticulum [Ca2]; INa, sodium current; ICa,L, L-type calcium current; INaCa, Na/Ca exchanger current; InsCa, Ca2-sensitive nonspecific cation channel current; Irel, Ca2 release current of the sarcoplasmic reticulum (Reference 39, American Journal of Physiology with permission).
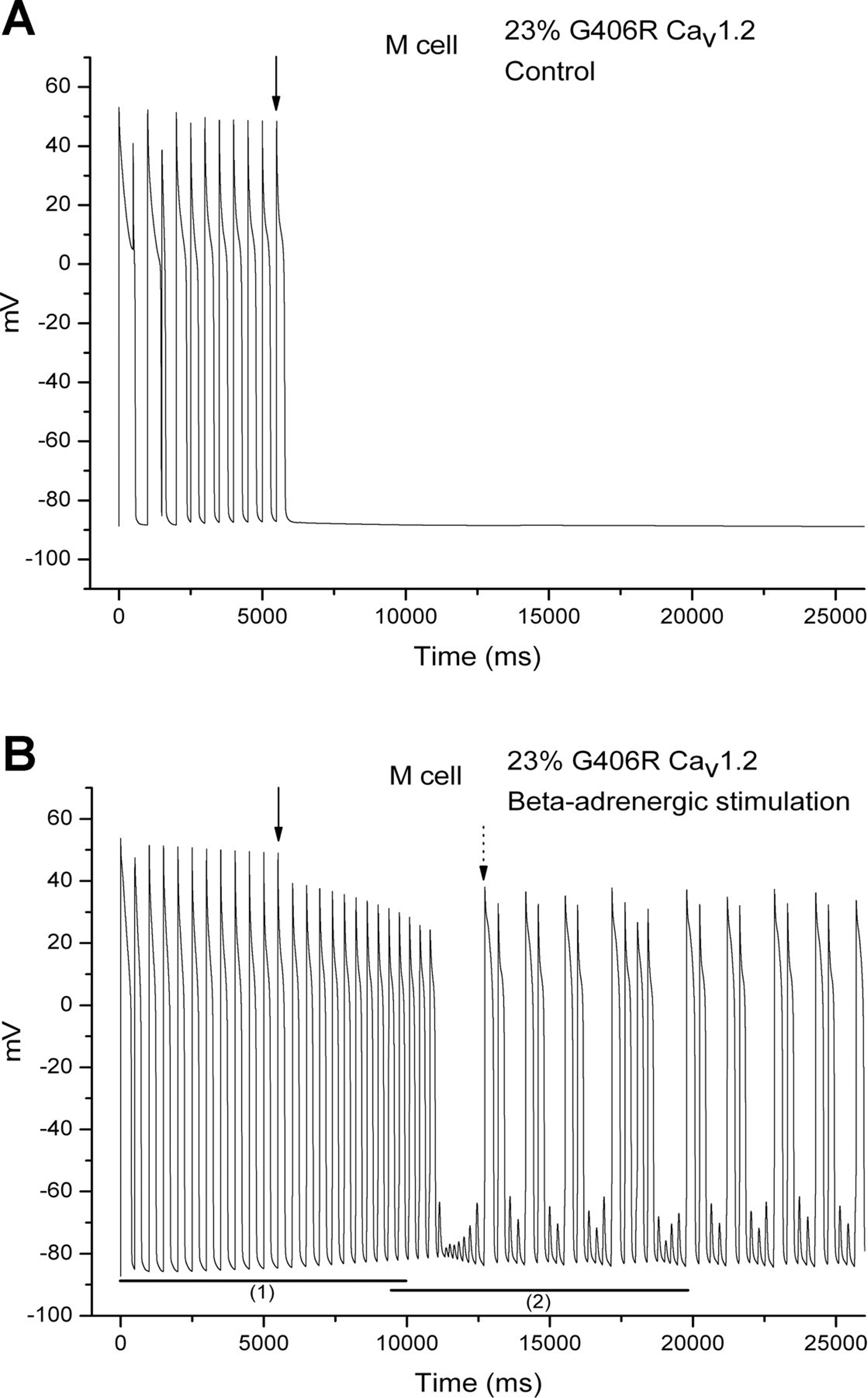
Effects of targeted therapy on BAS-enhanced inducibility of sustained DADs and DAD-mediated triggered activity in the ventricular myocyte containing 23% of G406R Cav1.2 channels. Effects are shown of 10 (A), 20 (B), and 40% (C) reduction of individually targeted currents, ICa,L, IUP, Irel, and INaCa, on the induction of sustained DADs and DAD-mediated triggered activity under the influence of BAS, as shown in Figure above. The solid arrow denotes the last (12th) pacing stimulus (Reference 39, American Journal of Physiology with permission).
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
Building on the success of our research on Timothy syndrome (TS), we concurrently investigated another hereditary arrhythmia: catecholaminergic polymorphic ventricular tachycardia (CPVT). Primarily associated with defects in the ryanodine receptor 2 (RYR2) or calsequestrin 2 (CASQ2) genes, CPVT is also a potentially fatal disease, mainly affecting young people (Reference 73).
Unlike TS, CPVT was characterized by a distinctly different subcellular mechanism, ultimately leading to intracellular calcium overload. This overload generated delayed afterdepolarizations and triggered activity while also amplifying the transmural dispersion of repolarization and enhancing reentrant substrates, thereby promoting reentrant arrhythmias. β-adrenergic receptor stimulation exacerbated these effects, triggering life-threatening ventricular arrhythmias and sudden death (Ref. 73). From an electrocardiographic perspective, CPVT did not affect action potential duration (APD) or QT interval, which distinguished it from TS.
Clearly, in TS, the resultant increase in L-type calcium channel current (ICa, L; Cav1.2) was inherently involved in the cellular processes of depolarization and repolarization, thereby affecting the action potential duration (APD) and ECG’s QT interval. In contrast, the defects in CPVT resided in subcellular structures (such as RYR2 and CASQ2), which did not influence cellular depolarization and repolarization and, therefore, exerted no effect on the action potential duration (APD) and the ECG’s QT interval.
More importantly, we further discovered that a combination of β-adrenergic receptor blockers (such as atenolol) and calcium channel blockers (such as verapamil) may have effectively reduced the L-type calcium current (ICa, L) and have suppressed the occurrence of ventricular tachyarrhythmias in CPVT patients.
Understanding the Mechanisms of Arrhythmia: Reentry and Triggered Activity
From a cellular electrophysiological perspective, intracellular calcium overload could induce early or delayed afterdepolarizations (EADs and DADs), leading to triggered activity and manifesting as arrhythmias. However, as described in our TS and CVPT simulation studies (references 37, 73), intracellular calcium overload could also promote the formation of reentrant substrates. Specifically, with increasing intracellular calcium overload, triggered activity could be induced, and transmural dispersion of repolarization could also be increased, creating reentrant substrates and promoting the likelihood of reentrant arrhythmia mechanisms. β-adrenergic receptor stimulation further exacerbated these phenomena.
Several years later, using cellular and 2-D tissue simulations, other researchers confirmed that DADs could generate triggered activity in tissue (reference 40). However, the propagation of triggered activity could lead to slow conduction and unidirectional block in neighboring regions with subthreshold DADs, resulting in reentrant arrhythmias. They attributed the occurrence of the latter two situations to the reduction of Na channel availability and the increase of gap junction coupling caused by subthreshold DADs, as well as increased tissue heterogeneity (reference 41). Additionally, researchers later created a TS gene knock-in swine model (i.e., knock-in swine model with CACNA1C Gly406Arg mutation) (reference 74) to demonstrate the existence of functional reentrant substrates through electroanatomical mapping, which was related to the decrease in peak INa current.
Shifting Gears: The Promise of iPS Cells
When planning further research projects, we learned about the groundbreaking research of Shinya Yamanaka (山中伸弥) (Kyoto University [京都大學]) – mature cells can be reprogrammed into pluripotent stem cells (induced pluripotent stem cells [iPS cells]) (Ref. 75). Specifically, he and his team successfully reprogrammed adult human dermal fibroblasts into a pluripotent state by introducing four defined transcription factors – Oct3/4, Sox2, Klf4, and c-Myc, generating induced pluripotent stem cells (iPS cells). These human iPS cells exhibit characteristics similar to human embryonic stem cells, including morphology, gene expression, and the ability to differentiate into various cell types. This achievement had opened up new possibilities for personalized medicine and disease modeling while avoiding the ethical issues associated with the use of human embryos. It further indicated that human iPS cells provide a powerful tool for regenerative medicine, establishing a new milestone in the study of patient-specific disease mechanisms and drug discovery. Shinya Yamanaka (山中伸弥) also won the Nobel Prize for this research work (2012 Nobel Prize in Physiology or Medicine). Recognizing this paradigm shift, we decided to end our research using computational simulation modeling and we wrote a review article to update our knowledge on inherited ion channelopathy (Ref. 76).
The Kuo-Ting Lee Memorial Library (國鼎分館) and Optoelectronics Building (國鼎光電大樓): Enlightenment
National Central University (國立中央大學)has the Kuo-Ting Lee Memorial Library (國鼎分館) and the Kuo-Ting Lee Optoelectronics Building (國鼎光電大樓) to commemorate Mr. Kuo-Ting Lee (李國鼎 [1910-2001]). Mr. Lee graduated from the Department of Physics at the predecessor of National Central University, Dongnan University (東南大學Nanjing, China). He pursued graduate studies at the University of Cambridge in the UK. In Taiwan, he served as Minister of Economic Affairs (經濟部長), Minister of Finance (財政部長), and Presidential Advisor, known as “Taiwan’s Financial Architect.” When Morris Chang (張忠謀先生), the founder of TSMC (Taiwan Semiconductor Manufacturing Company [台積電]), accepted the Kuo-Ting Lee Memorial Award (李國鼎紀念獎), he said, “Without Kuo-Ting Lee, there would be no TSMC.” In 1985, after heart surgery, he visited San Francisco and came to the UCSF outpatient clinic for me to evaluate his physical condition. I was aware of his contributions to Taiwan’s economic, financial, and technological development. I felt incredibly honored by his visit. When I returned to Taiwan, I specifically visited him at the Executive Yuan Science and Technology Building (行政院科技大樓) in Taipei. He and his wife were devout Christians. I was deeply moved by his advocacy of the “concept of the individual and the group (群己觀念)” and his patriotism and love for the people in Taiwan. I believe that we medical professionals should also take him as a role model and dedicate ourselves to serving the country and society.
Along the line, I remember a few notable people I have encountered. First, the master of the medical and pharmacology realms, Academician Lee Cheng-yuan (李鎮源院士 [1915-2001]), is a good example. After the 1990s, he began to step out of the research room and into Taiwanese society, actively participating in various democratic movements, leading the establishment of the “100 Action Alliance「100行動聯盟」” and successfully abolishing the undemocratic and unjust Article 100 (100刑條). This achievement is no less than his achievements in snake venom pharmacology research. Academician Lee Cheng-yuan once nominated me as a candidate for the dean of the National Taiwan University College of Medicine in 1998, which was a great honor for me. Academician Lee’s tireless efforts for social justice and mentoring of juniors are exemplary in my life.
Secondly, during my time in Taiwan, one day, I received a call from Professor Zhen-Yang Chen (陳振陽教授) of the National Taiwan University College of Medicine, asking me to visit the intensive care unit of Taipei Medical University Hospital (台北醫學大學附設醫院) to see the famous music master Tyzen Hsiao (蕭泰然先生). Mr. Hsiao lay weakly in bed, suffering from severe cardiovascular disease complicated by systemic bacterial infection. After understanding his condition, I quickly discussed some directions for improvement with the care team. I didn’t have much hope in my heart, but I didn’t know where the courage came from, thinking it was a farewell, and I even asked him to hold hands with me to pray. My prayer was unusually smooth and fluent. About a year later, to my surprise, he was conducting a symphony orchestra in Los Angeles again. Later, I learned that he and his wife were also devout Christians. Mr. Hsiao’s achievements in music and his life that glorified God and benefited people were also worthy of my learning.
Recently, we are all saddened by Ms. Monghui Peng (彭蒙惠女士)’s passing. Ms. Monghui Peng’s real name is Doris Marie Brougham (1926-2024). She was born in Seattle, Washington, USA. From a young age, she was determined to spread the gospel of Christ. After graduating from the University of Washington in 1948 with a major in Far Eastern Studies, she chose to go to China to preach the gospel. At that time, her Chinese name, “Monghui (蒙惠),” was taken from the meaning of being blessed by God. In 1951, she fled from war-torn China to Taiwan, and since then, she had dedicated her life to the people of Taiwan for more than 70 years. In 1962, she founded Studio Classroom (空中英語教室), and in 1963 produced the gospel TV program “Heavenly Melody (天韻歌聲),” with the Heavenly Melody Choir holding gospel music concerts in 36 countries around the world. When I was at NCKU, she and her group had a live play at our auditorium, and later, we signed a contract with Studio Classroom to broadcast her programs to students every day. A few years ago, she officially became a Taiwanese citizen. Her passing is a significant loss to our country, Taiwan.
Speaking of Christian missionaries, I cannot help but think of the late Pastor Shiyuan Kou (寇世遠牧師 [1920-1993]), who was under my care for his medical problems during my tenure at Stanford University. To my knowledge, Pastor Kou was born in Fuzhou, Fujian (福建省福州市), China. In 1952, he was baptized as a Christian at the Taipei Assembly of Churches (教會聚會所). Since then, he had devoted his life to spreading the gospel, glorifying Christ, and saving people from suffering. He served in several Christ’s Houses ( 基督之家) he had founded and, every Saturday afternoon, broadcast the popular TV program “Between Heaven and Man《天人之間》. Due to his strong desire to spread the gospel, he went to China against my medical advice in 1993. After returning to San Francisco, he was in severe heart failure and died after my failed resuscitation efforts at the emergency room. Pastor Kou’s life and dedication to his mission reminded me of the movie “Mission,” which depicts a missionary’s loyalty and dedication to God, as well as the sacrifices they make for the people he served.
In essence, as medical service or medical research professionals, in addition to Academician Cheng-Yuan Lee (李鎮源院士), nonmedical professionals like Advisor Kuo-ting Lee (李國鼎資政), musician Tyzen Hsiao (蕭泰然音樂家), educator Ms. Monghui Peng (彭蒙惠教育家), and Pastor Shiyuan Kou (寇世遠牧師), their dedication to society and the country are all role models in our life journey.
Mentoring the Next Generation
As Chair Professor, I initiated and sponsored an annual retreat for all faculty and students held in a suburban setting. This event, inspired by a similar program I implemented at National Cheng Kung University, aimed to foster a collaborative and supportive environment for faculty development. However, at National Central University, we prioritized the progress of student research projects. Students presented their preliminary findings, receiving valuable feedback and guidance from faculty members in a relaxed atmosphere. The overwhelmingly positive response from both faculty and students affirmed the retreat’s value, making it an eagerly anticipated event each year.
Before retiring, I shared my experiences with students in a talk titled “What are students’ current responsibilities in Taiwan?” (https://youtube.com/watch?v=JSnoO1L-kmA&feature=sharec and profiles.stanford.edu/ruey-sung).
It is gratifying to see many students continuing their studies at other academic institutions such as National Taiwan University, National Cheng Kung University, National Yang-Ming University, Tsinghua University, etc. A few years later, one such student, Ming-Wen Chang (張明雯), even went on to earn a Ph.D. at the University of Maryland and is now pursuing postdoctoral training at Yale University. She focuses on messenger RNA research and dedicates herself to new drug discovery, research, and developmen

Chair Professor Ruey Sung with faculty members at the Institute of Life Sciences. In right photo, Associate Professor Roland (羅南德, left 1st) and Professor Shelly Huang (黃雪莉,right 1st) .




The yearly research retreat for students and faculty sponsored by Chair Professor Ruey Sung.




Retirement farewell party for Chair Professor Ruey Sung, June 2012 (left lower, Professor Shelly Huang [黃雪莉], Chair, Institute of Life Sciences; right lower, Professor Cheng Chung Lee [李正中], Dean, College of Engineering).

References
(72) Best JM, Kamp TJ. A sympathetic model of L-type Ca2+ channel-triggered arrhythmias. American Journal of Physiology-Heart and Circulatory Physiology, 2010;298:H3-4.
(73) Sung RJ, Lo CP, Hsiao PY, Tien HC. Tackling intracellular calcium cycling in catecholaminergic polymorphic ventricular tachycardia. A theoretical investigation. American Journal of Physiology-Heart and Circulatory Physiology, 2011;301:H1625-H638.
(74) Porta-Sánchez A, Mazzanti A, Tarifa C, Kukavica D, Trancuccio A, Mohsin M, Zanfrini E, Perota A, Duchi R, Hernandez-Lopez K, Jáuregui-Abularach ME, Pergola V, Fernandez E, Bongianino R, Tavazzani E, Gambelli P, Memmi M, Scacchi S, Pavarino LF, Franzone PC, Lentini G, Filgueiras-Rama D, Galli C, Santiago DJ, Priori SG. Unexpected impairment of INa underpins reentrant arrhythmias in a knock-in swine model of Timothy syndrome. Nature Cardiovascular Research, 2023;2:1291-1309.
(75) Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007;131:861-872.
(76) Hsiao PY, Tien HC, Lo CP, Juang JM, Wang YH, Sung RJ. Gene mutations in cardiac arrhythmias: a review of recent evidence in ion channelopathies. Application of Clinical Genetics, Dovepress, 2013;6:1-13.
Closing Remarks with Reflection
As a clinician specializing in cardiology and clinical cardiac electrophysiology for over five decades, I cannot overstate the significance of continuous learning. My mentor, the late George Magnin from the University of Wisconsin, strongly advocated for two to three years of general medicine training in holistic care before entering any specialty. This commitment to ongoing education has not only provided a solid foundation for patient care but also served as a constant source of inspiration and motivation throughout my career.
Mastery of Medical Principles
Beyond achieving accurate diagnoses, clinicians must master essential medical principles, particularly the complex interactions between organs and systems and the effects of medications. This mastery is crucial for delivering comprehensive care and instills a sense of competence and confidence in our practice. Continuous learning keeps us motivated and competent in our field. For example:
- Atherosclerotic Coronary Heart Disease: Both hyperthyroidism (which increases metabolic rate) and hypothyroidism (which raises oxygen affinity in red blood cells [the oxyhemoglobin dissociation curve]) can exacerbate myocardial ischemia. It is vital to maintain patients in a euthyroid state.
- Awareness of Silent Cardiac Events: It is crucial to recognize that syncope or cardiac arrest may occur due to silent episodes of myocardial ischemia or cardiac arrhythmia, which can present without any symptoms such as chest pain or palpitations. Similarly, a lack of negative physical findings or abnormal test results does not necessarily rule out the presence of these serious conditions.
- Kidney Function: Hypotension is commonly seen in patients with heart or liver failure, particularly while on diuretic therapy. NSAID analgesics are contraindicated, and the use of RAAS inhibitors, antibiotics, and contrast media should be carefully assessed to prevent acute renal injury (Understanding the steep curve relationship between serum creatinine [Cr] and glomerular filtration rate [GFR]).
- Acid-Base Balance: Identifying underlying causes is essential for timely correction. For example, respiratory failure can lead to primary respiratory acidosis with secondary metabolic alkalosis, while uncontrolled diabetes may develop primary metabolic acidosis (ketoacidosis) accompanied by secondary respiratory alkalosis.
- Physical Examination: A thorough physical examination is essential for identifying critical signs of cardiovascular issues. For instance, the presence of elevated neck jugular veins with characteristic CV waves suggests right-sided heart failure. A systolic murmur heard at the left sternal border that intensifies during inspiration indicates tricuspid regurgitation. Conversely, if the murmur increases in intensity during the Valsalva maneuver (when intrathoracic pressure rises and venous return decreases), it may indicate left ventricular outflow tract obstruction, such as hypertrophic obstructive cardiomyopathy (HOCM).
- Differentiating Conditions and Collaborative Care: A skilled physician is adept at distinguishing between active and inactive medical conditions, managing cases within their area of expertise, and referring patients to the appropriate subspecialists when necessary. My experience in a group practice within an academic environment has allowed me to collaborate closely with colleagues, facilitating effective referrals that often lead to positive outcomes and gratifying feedback.
- Medication Considerations: Understanding the pharmacokinetic and pharmacodynamic properties of medications is vital, particularly regarding potential drug interactions. For example, the selection and dosage of anticoagulants must consider factors such as age, gender, liver and renal function, as well as interactions with other medications to minimize the risk of serious bleeding complications.
- Heart failure management: As left-sided heart failure progresses to right-sided heart failure and pulmonary hypertension develops, dyspnea may paradoxically decrease, creating a false sense of improvement. Early identification and treatment of left-sided heart failure are crucial for preventing pulmonary hypertension and its associated complications.
- Use of Defibrillator: Defibrillation is unsynchronized and does not require QRS complex detection. In contrast, cardioversion requires synchronization to the QRS complex, with energy levels adjusted based on the specific arrhythmia.
- The Human Element in Medicine: Ultimately, as physicians, we treat patients—individuals with unique stories—rather than merely interpreting ECGs, laboratory results, or diagnosing illnesses. It is essential to infuse every patient interaction with hope and positivity, acknowledging that miraculous outcomes can and do occur throughout our medical careers.
A Moment of Reflection: Learning from Adversity
In 1983, I sought to validate my discovery regarding the anatomical basis for dual atrioventricular (AV) nodal conduction by recruiting a 22-year-old patient diagnosed with AV nodal reentrant tachycardia. During an attempt at catheter ablation, we applied half of the electrical shock energy (the only energy source available at that time) required for His bundle ablation near the coronary sinus, which unfortunately led to cardiac tamponade and necessitated emergency surgery (unpublished case report). This challenging experience underscored the importance of learning from adversity; it instilled in me a profound sense of resilience that has empowered my future medical practice.
Reflecting on similar themes of perseverance and innovation, I recall John Swartz’s pioneering work in 1994 on catheter ablation for atrial fibrillation using radiofrequency energy. This complex procedure resulted in the unfortunate deaths of three out of ten patients (3/10). Despite his efforts to present these findings at the American Heart Association meeting, he faced challenges in publishing due to the high mortality rate associated with his initial results (Ref. 77). Nevertheless, Swartz’s contributions laid foundational work for the field of catheter ablation, which has since evolved significantly with advancements in instrument and technology (e.g., autonomous endocardial mapping, cryoablation, pulsed-field ablation, etc).
“Just like the Apollo 11 mission, these stories reveal that its triumphant moon landing was achieved through countless setbacks and the sacrifices of many.” Today, we recognize that Swartz’s early endeavors were crucial in shaping modern approaches to atrial fibrillation treatment. The evolution of catheter ablation techniques has transformed it into a highly effective treatment strategy, significantly improving patient outcomes over the years. His legacy reminds us that even amidst setbacks, innovation and dedication can lead to substantial advancements in medical practice.
A Lifelong Commitment: Decoding the Heart’s Rhythm
Learning and teaching have been central to my journey. During my fellowship at Jackson Memorial Hospital under Professor Agustin Castellanos Jr., I transitioned from static ECG analysis to dynamic arrhythmia research using intracardiac recordings. This exploration led to groundbreaking discoveries about dual AV nodal pathway physiology. We found that understanding the unique characteristics of these pathways can significantly improve the treatment of AV node reentrant tachycardia. My exploration continued at the University of California at San Francisco (UCSF), where I classified ventricular tachycardias in the electrophysiology laboratory (Refs 37,38), and my career later culminated at Stanford University, where I founded the Cardiac Electrophysiology and Arrhythmia Service, integrating research, education, and patient care. Administrative roles at Cathay General Hospital and National Cheng Kung University (NCKU) did not deter my commitment to cardiac electrophysiology. During a fortuitous opportunity at National Cheng Kung University, I delved into the study of inherited ion channel diseases through computational modeling. This research method allowed me to directly observe how different ion channel currents (Na+, Ca++, and K+) induce changes within cells and even smaller subcellular compartments, providing me with a deeper understanding of the mechanisms underlying electrocardiograms.”
As such, I am eager to share my journey with fellow healthcare professionals to inspire exploration within their fields. ECG interpretations must be contextualized within clinical practice as part of a ‘five-finger approach’ guiding informed patient care decisions. This approach involves considering the patient’s history, symptoms, physical examination, ECG findings, and response to treatment. It’s this comprehensive approach that empowers us to make informed decisions and provide the best care for our patients. I realize that each journey is unique and filled with obstacles. I am thankful for those who have supported me along the way. To conclude, I share a prayer by Rev. Karl Paul Reinhold Niebuhr (1892-1971) that has inspired me throughout my career:
“God, grant me the serenity to accept the things I cannot change, courage to change the things I can, and wisdom to know the difference.”
References:
(77) Swartz JF, Pellerseis G, Silvers J, Patten L, Cervantez D. A catheter-based approach to atrial fibrillation in humans. Circulation, 1994;90(Suppl I): 335 (Abstract).
ECG Cases
It illustrates my thinking process when I look at the ECG during my initial workup of the patient. I hope you find it helpful in taking care of your patients.
Inspirational images
It displays photographic images of art and the beauty of nature that resonate with positive thinking about life, encouraging medical students and young physicians never to give up pursuing their dreams.
Unchartered Journey
It describes my unchartered academic career from becoming an internist, cardiologist, and clinical electrophysiologist to a medical educator in different stages before retirement.
Contact
If you have further questions or have interesting ECGs that you would like to share with us, please email me.